
The impact of nutrition and lifestyle on chronic urinary bladder conditions: a literature review
Introduction
Background
You might have heard of the saying “you are what you eat”. This is reflected by the acknowledgment of the effect of nutrition on human health since ancient times. However, it was not until the 19th and 20th centuries that dietary management and supplements were integrated into the treatment of medical conditions. During the 1970s, numerous research studies tried to refine the management of urinary conditions by including diet in the therapy (1). More recently, there has been an increased attention towards the impact of tailored nutritional intake and lifestyle modifications in the enhancement of symptoms of chronic bladder conditions, including interstitial cystitis (IC), overactive bladder (OAB), chronic painful bladder syndrome (PBS) (stand alone or as part of a chronic pelvic pain syndrome), and post-traumatic bladder irritation (i.e., after bladder tumor resection and/or radio-/chemotherapy). Patients living with those chronic bladder conditions develop coping strategies to hide their bothersome symptoms such as urinary incontinence (UI) and nocturia. These symptoms subsequently negatively impact their quality of life in several aspects including their physical, social, and psychological (anxiety, negative self-image, and isolation) wellbeing (2,3).
The equation for a healthy lifestyle is simple: avoiding harmful products, committing to regular physical activity, and consuming a personalized diet based on your body’s needs. Any missing component of the formulation will not equate to the desired answer. Thus, following this holistic approach, along with the appropriate medical treatment, can significantly change the patient’s lifestyle, and in turn positively affect their wellbeing.
The way dietary products act on the urinary tract anatomy and physiology explains the relationship between them. Briefly, nutrients or its metabolites excreted in the urine can either act on urinary bladder urothelium directly, can modulate urinary properties (i.e., pH), or can reach the bladder muscle through the blood stream where they act at tissue level as anti-inflammatory or immune-boosting products. They may also act as neuromodulators of the sympathetic and parasympathetic bladder innervation. In certain chronic conditions such as IC, the glycosaminoglycan layer of the urothelial barrier is dysregulated as a result of abnormal protein expression, disruption of proteoglycans, tight junctions, cell adhesion proteins and bacterial defense molecules (4,5). This allows typically harmless dietary metabolites to migrate across the urothelium and act as noxious stimuli leading to symptoms such as sharp pelvic pain and dysuria (6). Similarly, dietary metabolites acting as stimuli in one organ can produce parallel physiological effects in neighboring organs through a mechanism known as organ “crosstalk” via integrated sensory pathways. One important example is the possible interaction between irritable bowel syndrome and OAB. Not only are these visceral organs anatomically close but they also share similar physiological roles, such as storing and excreting wastes from body, as well as their common innervation from spinal afferent pathways through convergence of pelvic afferents (7,8). Experimental evidence from an animal study involving single-unit bladder afferent nerve recordings has shown that colonic irritation directly sensitizes the mechanical and chemical receptive properties of bladder sensory C-fibers, with mastocytosis largely contributing to the long-term pelvic organ sensitization (9). Nutrition plays a major role in bowel function, and—given the above relationship—this may also be true for the bladder whereby colonic inflammation may result in severe bladder dysfunction (7).
In this review, we will highlight the evidence-based relationship between certain comestibles and lifestyle factors on chronic bladder conditions. The aim of this content is to allow the reader to make an informed decision by the end of the paper on how they would like to adjust their way of living, particularly to the best version of themselves.
Rationale and knowledge gap
It is known that consuming harmful products such as cigarettes or ingesting fast food can harm our health. However, there are a few papers that integrate evidence-based information on the combined effect of lifestyle and diet on common bladder conditions like OAB and IC. This comprehensive review allows the patient to understand the mechanism behind this relationship and choose to apply it to their daily life.
Objective: “key question”
“What is the medical evidence behind lifestyle and dietary modifications on chronic bladder conditions and how exactly will it impact the symptoms of chronic bladder conditions” is the key question that the reader will conclude while exploring this review. The objective is to collect and review the available evidence in the literature on the topic and present it in a comprehensive review to help raise an understanding of the mechanisms and awareness of the ameliorating role of nutritional science in patients with chronic bladder conditions. We present this article in accordance with the Narrative Review reporting checklist (available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-39/rc).
Methods
A literature review was conducted in April 2020 and updated in November 2023 following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (10) (Figure 1).
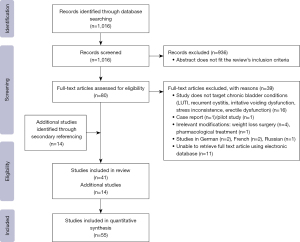
When searching PubMed, a combination of Medical Subject Headings (MeSH) search terms was used and they included all of the above-mentioned bladder conditions, as well as “bladder”, “lower urinary tract symptoms”, “nocturia”, “frequency”, “urgency”, “dysuria”, “chronic pelvic pain”, “irritable bowel syndrome”, “bladder pain” in combination with “nutrition”, “nutrients”, “diet”, “lifestyle”, and “herbal medicine”. Studies that cover the nutritional approach of irritable bowel were evaluated to assess a relation with bladder conditions of mutual symptoms.
Study and data selection process: after an initial selection of studies based on the title and abstract, nominated studies were further scrutinized for full text screening. Irrelevant studies that did not fit the eligibility criteria were excluded. Studies that were selected for full article screening were evaluated. Applicable full text articles were included in the qualitative synthesis. Reference lists from each retrieved full text article were evaluated through secondary referencing, and applicable studies were added to the qualitative synthesis. The data was collected by one reviewer under the supervision of co-reviewers.
Eligibility criteria: all studies published in English or translated to English in a listed peer reviewed scientific paper or international reputed journal were included. Published web communications by a reputed health organization or patient organization were also included. Case reports and studies that did not meet these criteria were excluded.
There was no statistical analysis conducted in this study. Qualitative and quantitative information collected from included studies were summarized and organized in specially designed tables. Only English studies were included in this study which introduced language bias. There are no ethical considerations in this paper. The search strategy is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 10th of April 2020 (updated in November 2023) |
| Databases and other sources searched | PubMed, Google Scholar |
| Search terms used | “Interstitial cystitis”, “overactive bladder”, “painful bladder syndrome”, “bladder”, “lower urinary tract symptoms”, “nocturia”, “frequency”, “urgency”, “dysuria”, “chronic pelvic pain”, “irritable bowel syndrome”, “bladder pain” in combination with “nutrition”, “nutrients”, “diet”, “lifestyle”, and “herbal medicine” |
| Timeframe | January 1984–November 2023 |
| Inclusion and exclusion criteria | All studies published in English or translated to English in a listed peer-reviewed scientific paper or international reputed journal were included. Published web communications by a reputed health organization or patient organization were also included. Case reports and studies that did not meet these criteria were excluded |
| Selection process | Data was collected by a single author under the supervision of the co-authors. No consensus was required |
Results
Initially, 1,016 articles were identified. Following screening by title and abstract, 80 studies were identified for full text evaluation. Finally, 39 articles were included in this review, in addition to 14 studies retrieved from secondary referencing of these selected studies. Updated search in 2023 included two more systematic reviews with similar evidence identified in this review. The PRISMA flow diagram (Figure 1) was used to record and summarize the results of the data selection process. There is no discrepancy between abstract and main journal outcome.
Discussion
OAB
OAB is a symptom complex of urgency, with or without urgency incontinence, usually with increased daytime frequency and nocturia (11). It is a prevalent condition which affects approximately 11–16% of men and 13–17% of women aged ≥18 years (12,13). There are a variety of modifiable behavioral and lifestyle factors, both, protective or damaging that can affect bladder health and educating the people at risk is important for the prevention of development of OAB (14-16). Apart from pharmacotherapy, treatment of OAB includes behavioral interventions and lifestyle changes, which are collectively considered first-line treatments for OAB symptoms, as well as diet modifications and herbal medications.
Effect of lifestyle on OAB
Behavioural modifications
Behavioral modifications altering habits that may alleviate bladder symptoms and promote bladder health such as smoking cessation as well as diet, fluid intake, bowel, and weight management, and training techniques that involve teaching skills that promote symptom control in bladder dysfunction (17). This includes counseling the patient that the bladder should be emptied every 3 to 4 hours, and that it is not necessary to void with every sensation of bladder fullness, considering the pathophysiology of OAB which involves oversensitivity of the detrusor muscle to bladder signals. Patients are advised to keep bladder diaries (18). Training techniques include simple urgency control and suppression techniques, bladder training, and multicomponent behavioral training. Simple urgency control and suppression techniques can be applied by performing general relaxation techniques and slow deep breathing that decreases the intensity or urgency and relaxes the bladder. Distracting techniques like sudoku puzzles and self-motivational phrases like “I can” are also effective (17,19). Similarly, bladder training aims to restore normal bladder function by combining a progressive voiding schedule and teaching techniques to suppress urgency. It is suitable for motivated patients has been showed to resolve urinary urge-incontinence by 12% to 73% (20). In multicomponent behavioral training, which is composed of techniques known as pelvic floor muscle (PFM) contractions also called Kegels, the patient is taught to locate their striated skeletal PFM around the urethra and contract them in order to relax the detrusor muscle, aiming to suppress urgency, control incontinence and restore normal voiding interval (14). A combination of correctly taught bladder training, PFM exercises, fluid restriction, and electrical stimulation showed a significant reduction in frequency and residual volume in patients with idiopathic detrusor instability while increasing voiding interval, flow rate, and maximum capacity (21). Finally, poor lifestyle factors that are linked to diabetes and obesity, such as lack of exercise can contribute to onset of OAB (22,23). The evidence is conclusive that lifestyle modifications like habit changes and certain training techniques like PFM exercises can improve OAB symptoms.
Weight and obesity
Obesity is a modifiable risk factor for UI which can be a symptom of OAB. Recent studies discuss the benefits of nonsurgical weight loss on reducing symptoms of OAB, particularly urinary urge-incontinence. Obesity increases the risk of onset of OAB in women (23). In a study carried out on 338 obese women, a 6-month weight loss program (diet, exercise, and behavioral modification) was initiated. The average weight loss in the intervention group was 8.0%. After 6 months, the intervention group showed a mean decrease of 47.4% in the total number of incontinence episodes per week as compared to the control group with 28.1%. There was a 42.4% decline in the frequency of urge incontinence episodes compared to 26.0% in the control group. However, these differences were not statistically significant (24). In a similar study, it was concluded that 5% to 10% of weight loss was sufficient to significantly improve UI. Thus, weight loss should be considered as the initial treatment for incontinence in overweight women (25). Moreover, because diabetic neuropathy, a complication of diabetes, may lead to OAB, obese prediabetic women are encouraged to lose weight to reduce the risk of development of type 2 diabetes. The lower prevalence of UI resulting from lifestyle modifications, particularly weight loss, provides a powerful motivator for prediabetic women to reduce their weight (26).
Smoking and alcohol consumption
Smoking has been associated with symptoms of OAB, with an increased chance of incontinence in previous and current female smokers (27,28). Another study showed that smoking increased the 1-year incidence of OAB in women (23). Similarly, smoking in men has been linked with OAB and prostatic enlargement (28). However, smoking showed no association with the onset of OAB in men, but an increased risk in females by factor 1.44 (23,29). Notably, studies on cats show nicotine induced detrusor overactivity (30).
In a study to investigate the onset of OAB in male patients, beer consumption showed a significant protective role for the onset of OAB at all levels of intake compared to people who never drank beer (29). In contrast, another study reported that alcohol increases the risk of lower urinary tract symptoms (LUTS) (31) thus studies are inconsistent.
Effects of nutritional intake on OAB
Comestibles
A balanced diet is the key to a healthy body. More particularly for your bladder health, consuming large quantities of potato showed an increased risk of onset of OAB (P<0.05) (29). Similarly, in women, consumption of carbonated drinks increased the risk of OAB while vegetables, bread, and chicken (preferably twice or more a week) reduced the risk (23).
Moving on to the crowd’s favourite: caffeine. There is a strong evidence that caffeine ingestion at a dose of 4.5 mg/kg produced diuretic effects with an increased flow rate and voided volume, increasing urgency and frequency symptoms in OAB (32). Patient education about this concept resulted in significant reduction in caffeine intake (P<0.0001) in patients with LUTS and, in turn, significantly reduced frequency (P=0.037) and urgency (P=0.002) (33).
Additionally, a study carried out on Korean women showed a significantly higher carbohydrate content in women with UI compared to a control group (P=0.041) (34). Poor evidence revealed that high dietary levels of protein (P=0.03) and potassium (P=0.05) decreased the risk of onset of OAB, as did an increased intake of niacin (P=0.13) and vitamin B6 (P=0.08) (35). In a study on Malaysian elderly, increased dietary monosaturated fat and plasma triglyceride levels decreased the risk of UI (36).
Even though 60% of our body is made up of water, consuming fluids at a moderate rate is recommended to reduce unwanted side effects. Drinking an average of >3,700 mL/day of fluids leads to a higher voiding frequency and risk of UI compared to an intake of <2,400 mL/day. Research shows that nocturia in the context of OAB can be managed by the ingestion of fruits and vegetables while avoiding caffeine and high-salt diet (37). Likewise, vegetable intake in patients with type 2 diabetes mellitus (T2DM) showed reduced nocturia (≥2 voids/night) in men as well as reduced severe nocturia (≥3 voids/night) in females (38).
Finally, dietary phytoestrogens, typically found in soybeans, tofu, and linseed, in low and high intake levels, showed no association in the development or prevention of urge incontinence in midlife women transitioning to menopause (39). Pre-treatment with epigallocatechin-3-gallate, a major catechin found in green tea, cranberry, and nuts, can prevent OAB induced by metabolic syndrome/ovarian hormone deficiency by reducing inflammation, improving storage function, and in turn decreasing bladder overactivity (40). The effects of comestibles on OAB are summarized in Table 2.
Table 2
| Foods/beverages that exacerbate symptoms of OAB | Foods/beverages that alleviate symptoms of OAB |
|---|---|
| Carbonated drinks (sparkling water, energy drink) | Fruits (banana, apple, coconut, watermelon) |
| Caffeinated beverages (coffee, tea) | Vegetables (broccoli, celery, asparagus, kale) |
| Chocolate | Fiber rich foods (lentils, beans, oats, almonds) |
| Citrus fruit | Protein (fish, chicken, eggs) |
| Spicy food | |
| Honey | |
| Raw onion | |
| Tomato and tomato products (e.g., ketchup) | |
| Artificial sweetener (saccharin, aspartame) | |
| Salty food (chips, salted nuts) | |
| Cranberry |
OAB, overactive bladder.
Vitamins and minerals
Vitamins and minerals in the body have a very integral role in several metabolic functions such as converting food into energy and repairing cellular damage. Recent studies have been focusing on vitamin D and how it affects the normal bladder physiology. In one study carried out by the National Health and Nutrition Examination Survey, involving 1,881 women participants, symptomatic patients with UI measured lower mean vitamin D levels in their body. In addition, vitamin D deficiency in geriatric patients shows association with OAB and increased severity of UI, particularly urgency incontinence as a result of its effects on smooth and straited smooth muscle function (41). High intake of vitamin D decreases risk of onset of OAB (P=0.008) (35,42). Furthermore, higher levels of Vitamin D (>30 ng/mL) have been proven to show protective function in younger and older women with or without UI (43). This finding is further supported by a randomized control trial that was carried out on postmenopausal women with LUTS. They were treated with high dose of vitamin D (20,000 IU of vitamin D twice a week) in comparison to the control group treated with standard vitamin D dose. After 1 year of treatment, there was a statistically significant reduction in improvement of LUTS, including reducing the severity of urine incontinence, in comparison to the control group (P<0.05) (44). In a cohort study, seasonal variation of vitamin D levels was demonstrated. During the winter season, patients presented with lower vitamin D levels and consequently, aggravated LUTS and increased severity of OAB symptoms as well as a greater impact on the quality of life. However, prostate volume, maximum urinary flow rate (Qmax) and post-void residual (PVR) urine volume were not affected (45).
One possible theory is the anti-inflammatory action of vitamin D supplements. This was demonstrated by lowering erythrocyte sedimentation rate (ESR) levels in people treated with vitamin D, thus, possibly, reducing effects of chronic inflammation in the bladder (45).
Vitamin D receptor agonist known as elocalcitrol showed an inhibition in the RhoA/Rho kinase signaling pathway and upregulation in the Ca2+ entry through L-type channels which modulates bladder contractility (46). Vitamin D and its analogs were shown to cause calcium desensitization thereby relaxing smooth muscles (42).
On the other hand, water-soluble vitamin C can also produce changes in the bladder. Baseline dietary intake of vitamin C from fruits and vegetables was associated with lower chance of progression of daytime storage symptoms in men and urgency symptoms in women.
Inversely, excessive vitamin C supplements has shown worsening in daytime urinary storage problems in women (47). This is due to the increased level of vitamin C byproduct, ascorbate, in the urine resulting in increased acidity of urine composition leading to OAB symptoms (48).
Herbal treatments
Herbal medicine has shown an increased demand as a treatment for OAB. The World Health Organization states that approximately 80% of the world’s population currently uses herbal medicine for some aspect of primary health care, with a higher percent of women than men (49,50). In a 12-week study carried out on 117 women, the effectiveness of Granu Fink Femina on OAB was investigated. It is a German-based herbal medication containing high concentration of plant phytosterols including seed oil from uromedic pumpkin, rhus aromatica bark extract, and humulus lupulus cone extract. The combination of those products acts on the beta3-adrenoceptor agonists in the detrusor muscle to strengthen and calm the bladder with minimal side effects. Results showed statistically significant decrease in urinary frequency as well as decrease in frequency of leakage and used pads from the 1st week of initiation of treatment. In addition, the quality of life of the patient showed improvement from the 1st week of treatment and continued to progress until the 12th week, and it also showed excellent tolerability by the patients (51). A recommended dose of 500 mg was shown to benefit patients with mild to moderate LUTS. The evidence confirms that recommended herbal medications can improve bladder frequency and storage symptoms.
IC/PBS
IC or PBS is a common cause of chronic pelvic pain, with suggestive symptoms found in 2.7–6.5% of women in the USA (52). Symptoms include frequency, urgency, dysuria and lower abdominal, bladder, vaginal, urethral, or perineal pain, in the absence of bacterial cystitis. It is a difficult condition to treat, however, certain management plan including dietary and behavioral changes can help improve symptoms (53,54). Medications and surgery can be considered should these conservative measures fail (45).
Effect of nutritional intake on IC/PBS
Comestibles
Consuming bladder friendly food and beverages can limit bladder inflammation and irritation. In a questionnaire designed to explore the relationship between food/beverages and bladder symptoms, 90.2% of participants reported exacerbation in IC/PBS with certain nutrients (55). Recent studies state that acidic food like citric fruits worsen IC symptoms (56) while alkaline food like sodium bicarbonate and acid neutralizing food like calcium glycerophosphate improve symptoms (57). Moreover, high potassium levels (sweet potato, milk) may be linked to exacerbation of bladder pain (58), however only studies with small sample size were carried out and further surveys do not show alteration in IC symptoms with potassium intake. Thus, results are inconclusive. Histamine containing food (like eggplant) can exacerbate IC by increasing mast cells and inflammation (1). Similar to OAB, caffeine can aggravate IC symptoms in the same mechanism (59). In an animal study, protein deficiency showed an increased risk in the development cyclophosphamide (CP) induced hemorrhagic cystitis (HC) in rats, stressing on the importance of nutritional assessment and supplementation prior to CP treatment (60). Likewise, patients with compilation of pelvic radiotherapy, known as hemorrhagic radiation cystitis (HRC), presented with higher grades of hematuria associated with lower serum nutrients like albumin, prealbumin, zinc, selenium, and essential fatty acids. It is known that a good nutritional status is required for wound healing, which enhances the efficacy of hyperbaric oxygen therapy (HBOT). Thus, pre-treating HRC patients with dietary supplements prior to HBOT would maximize healing of bladder mucosa (61). The effects of comestibles are summarized in Table 3.
Table 3
| Foods/beverages that are most bothersome to patients with IC/PBS | Foods/beverages that are least bothersome to patients with IC/PBS |
|---|---|
| Cranberry juice, carbonated water, green tea | Apples, pears, blueberries |
| Coffee (caffeinated and decaffeinated) | Mushroom, carrot, corn |
| Alcohol (beer, red and white wine, champagne) | Milk, water, chamomile, peppermint |
| Acidic fruit (lemon, pineapple, kiwi) | Corn and oat bread, rice |
| Salad dressing, vinegar | Eggs, beef, fish, lamb |
| Chili pepper, onion, pickles, tomato (and products) | Oil, butter, nuts |
| Hotdog, smoked fish | Ricotta cheese, cottage cheese, ice cream |
| Chips and fast food | Popcorn, pretzels |
| Spicy food/Mexican, Thai, and Indian food | Custard, vanilla flavour pudding, and milkshake |
| Artificial sweetener, chocolate, candy | Homemade soup |
IC, interstitial cystitis; PBS, painful bladder syndrome.
Vitamins
CP, an antineoplastic drug, induces HC. In rats, treatment with antioxidant vitamin C and histidine showed improvement of HC by reducing free radical induced damage (62). Vitamin C inhibited histopathological changes of CP like inflammatory infiltration and hemorrhage in mucosa propria (63), while histidine reduced congestion, hemorrhage, edema, and leukocyte infiltration (64). HRC is normally worse in patients with lower serum levels of vitamins C, D, and B12 (61).
Effects of lifestyle on IC/PBS
Smoking and alcohol
In a study on women participants, an association was found between bladder pain and smoking, where tobacco was labeled as a modifiable risk factor (65). In a similar manner, alcohol irritates wounds in IC bladder, exacerbating the symptoms. The O’Leary-Sant Interstitial Cystitis Symptom and Problem Indices Questionnaire (ICN) survey reported that only 21% of IC patients can tolerate drinking wine without getting IC flares, with tequila being the most irritating.
Behavioral modifications and physiotherapy
Pelvic floor manual therapy for women with IC caused mild to moderate improved in 70% of patients, by decreasing the exaggerated PFM tone thus alleviating urgency and frequency (66). Stress management, relaxation strategies, and sleep hygiene also improve symptoms of IC (67).
Conclusions
This review explored the effects of dietary and lifestyle factors on bladder health, particularly on symptoms of chronic bladder conditions, and focused on modifications of these factors that lead to improved management of symptoms and enhanced bladder health. The key modifications include behavioral and lifestyle changes, which are considered first-line treatment. Finally, and with medical/nutritionist supervision, elimination of harmful diet to the urinary bladder can be implemented in patients to determine dietary irritants without limiting their nutritional intake.
Limitations of the review
Despite the maximal efforts in providing a comprehensive review that displays evidence published over the past few years, there are limitations that arise. Firstly, consideration of nutritional variations in different regions as well as diverse patient factors (age, gender, comorbidities, etc.) were not discussed in this review as it was beyond the scope of the search. Secondly, even though papers were collected from a wide range of publication dates (from the early 2000s to 2020), including more recent papers would have added further value to our review. Finally, following the exclusion criteria of omitting papers published in non-English language limited the evidence that could have been impactful from other international authors. We aim to explore those differences in the future and compile a broader and more up to date review.
Acknowledgments
In loving memory of Doctor Noor Buchholz, whose unwavering support and invaluable contributions greatly enriched this research. Doctor Buchholz played an instrumental role in shaping the course of this work and his legacy will forever inspire our scientific endeavors. This paper is dedicated to Doctor Buchholz in profound gratitude for his enduring guidance and impact on our academic journey.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Longhua Chinese Medicine for the series “Integrative Medicine Approaches to Common Urological Problems”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-39/rc
Peer Review File: Available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-39/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-39/coif). The series “Integrative Medicine Approaches to Common Urological Problems” was commissioned by the editorial office without any funding or sponsorship. N.B. served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Friedlander JI, Shorter B, Moldwin RM. Diet and its role in interstitial cystitis/bladder pain syndrome (IC/BPS) and comorbid conditions. BJU Int 2012;109:1584-91. [Crossref] [PubMed]
- Abrams P, Kelleher CJ, Kerr LA, et al. Overactive bladder significantly affects quality of life. Am J Manag Care 2000;6:S580-90. [PubMed]
- Basra R, Kelleher C. Disease burden of overactive bladder: quality-of-life data assessed using ICI-recommended instruments. Pharmacoeconomics 2007;25:129-42. [Crossref] [PubMed]
- Hauser PJ, Dozmorov MG, Bane BL, et al. Abnormal expression of differentiation related proteins and proteoglycan core proteins in the urothelium of patients with interstitial cystitis. J Urol 2008;179:764-9. [Crossref] [PubMed]
- Hurst RE, Moldwin RM, Mulholland SG. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology 2007;69:17-23. [Crossref] [PubMed]
- Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 2007;69:9-16. [Crossref] [PubMed]
- Grundy L, Brierley SM. Cross-organ sensitization between the colon and bladder: to pee or not to pee? Am J Physiol Gastrointest Liver Physiol 2018;314:G301-8. [Crossref] [PubMed]
- Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology 2005;128:1953-64. [Crossref] [PubMed]
- Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourol Urodyn 2010;29:77-81. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Abrams P, Artibani W, Cardozo L, et al. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn 2009;28:287. [Crossref] [PubMed]
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20:327-36. [Crossref] [PubMed]
- Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006;50:1306-14; discussion 1314-5. [Crossref] [PubMed]
- Burgio KL. Behavioral treatment options for urinary incontinence. Gastroenterology 2004;126:S82-9. [Crossref] [PubMed]
- Smith AL, Wein AJ. Contemporary management of overactive bladder. Postgrad Med 2012;124:104-16. [Crossref] [PubMed]
- Newman DK, Cardozo L, Sievert KD. Preventing urinary incontinence in women. Curr Opin Obstet Gynecol 2013;25:388-94. [Crossref] [PubMed]
- Wyman JF, Burgio KL, Newman DK. Practical aspects of lifestyle modifications and behavioural interventions in the treatment of overactive bladder and urgency urinary incontinence. Int J Clin Pract 2009;63:1177-91. [Crossref] [PubMed]
- Godec CJ. "Timed voiding"--a useful tool in the treatment of urinary incontinence. Urology 1984;23:97-100. [Crossref] [PubMed]
- Wyman JF, Fantl JA. Bladder training in ambulatory care management of urinary incontinence. Urol Nurs 1991;11:11-7. [PubMed]
- Wyman JF. Treatment of urinary incontinence in men and older women: the evidence shows the efficacy of a variety of techniques. Am J Nurs 2003;26-35. [Crossref] [PubMed]
- Al-Mulhim AA, Al-Gazzar SA, Bahnassy AA. Conservative treatment of idiopathic detrusor instability in elderly women. Saudi Med J 2002;23:543-5. [PubMed]
- McGrother CW, Donaldson MM, Thompson J, et al. Etiology of overactive bladder: a diet and lifestyle model for diabetes and obesity in older women. Neurourol Urodyn 2012;31:487-95. [Crossref] [PubMed]
- Dallosso HM, McGrother CW, Matthews RJ, et al. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int 2003;92:69-77. [Crossref] [PubMed]
- Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med 2009;360:481-90. [Crossref] [PubMed]
- Wing RR, Creasman JM, West DS, et al. Improving urinary incontinence in overweight and obese women through modest weight loss. Obstet Gynecol 2010;116:284-92. [Crossref] [PubMed]
- Brown JS, Wing R, Barrett-Connor E, et al. Lifestyle intervention is associated with lower prevalence of urinary incontinence: the Diabetes Prevention Program. Diabetes Care 2006;29:385-90. [Crossref] [PubMed]
- Bump RC, McClish DK. Cigarette smoking and urinary incontinence in women. Am J Obstet Gynecol 1992;167:1213-8. [Crossref] [PubMed]
- Koskimäki J, Hakama M, Huhtala H, et al. Association of smoking with lower urinary tract symptoms. J Urol 1998;159:1580-2. [Crossref] [PubMed]
- Dallosso HM, Matthews RJ, McGrother CW, et al. The association of diet and other lifestyle factors with the onset of overactive bladder: a longitudinal study in men. Public Health Nutr 2004;7:885-91. [Crossref] [PubMed]
- Koley B, Koley J, Saha JK. The effects of nicotine on spontaneous contractions of cat urinary bladder in situ. Br J Pharmacol 1984;83:347-55. [Crossref] [PubMed]
- Seim A, Hoyo C, Ostbye T, et al. The prevalence and correlates of urinary tract symptoms in Norwegian men: the HUNT study. BJU Int 2005;96:88-92. [Crossref] [PubMed]
- Lohsiriwat S, Hirunsai M, Chaiyaprasithi B. Effect of caffeine on bladder function in patients with overactive bladder symptoms. Urol Ann 2011;3:14-8. [Crossref] [PubMed]
- Bryant CM, Dowell CJ, Fairbrother G. Caffeine reduction education to improve urinary symptoms. Br J Nurs 2002;11:560-5. [Crossref] [PubMed]
- Lee JH, Lee HS. Nutrient intake and urinary incontinence in Korean women: A propensity score-matched analysis from the Korea National Health and Nutrition Examination Survey data. Int J Urol 2017;24:793-7. [Crossref] [PubMed]
- Dallosso HM, McGrother CW, Matthews RJ, et al. Nutrient composition of the diet and the development of overactive bladder: a longitudinal study in women. Neurourol Urodyn 2004;23:204-10. [Crossref] [PubMed]
- Eshkoor SA, Hamid TA, Shahar S, et al. Factors Related to Urinary Incontinence among the Malaysian Elderly. J Nutr Health Aging 2017;21:220-6. [Crossref] [PubMed]
- Alwis US, Monaghan TF, Haddad R, et al. Dietary considerations in the evaluation and management of nocturia. F1000Res 2020;9:F1000 Faculty Rev-165.
- Furukawa S, Sakai T, Niiya T, et al. Dietary intake habits and the prevalence of nocturia in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2018;9:279-85. [Crossref] [PubMed]
- Waetjen LE, Leung K, Crawford SL, et al. Relationship between dietary phytoestrogens and development of urinary incontinence in midlife women. Menopause 2013;20:428-36. [Crossref] [PubMed]
- Lee YL, Lin KL, Wu BN, et al. Epigallocatechin-3-gallate alleviates bladder overactivity in a rat model with metabolic syndrome and ovarian hormone deficiency through mitochondria apoptosis pathways. Sci Rep 2018;8:5358. [Crossref] [PubMed]
- Kilic MK, Kizilarslanoglu MC, Kara O, et al. Hypovitaminosis D is an independent associated factor of overactive bladder in older adults. Arch Gerontol Geriatr 2016;65:128-32. [Crossref] [PubMed]
- Digesu GA, Verdi E, Cardozo L, et al. Phase IIb, multicenter, double-blind, randomized, placebo-controlled, parallel-group study to determine effects of elocalcitol in women with overactive bladder and idiopathic detrusor overactivity. Urology 2012;80:48-54. [Crossref] [PubMed]
- Badalian SS, Rosenbaum PF. Vitamin D and pelvic floor disorders in women: results from the National Health and Nutrition Examination Survey. Obstet Gynecol 2010;115:795-803. [Crossref] [PubMed]
- Oberg J, Verelst M, Jorde R, et al. High dose vitamin D may improve lower urinary tract symptoms in postmenopausal women. J Steroid Biochem Mol Biol 2017;173:28-32. [Crossref] [PubMed]
- Yoo S, Oh S, Kim HS, et al. Impact of serum 25-OH vitamin D level on lower urinary tract symptoms in men: a step towards reducing overactive bladder. BJU Int 2018;122:667-72. [Crossref] [PubMed]
- Morelli A, Squecco R, Failli P, et al. The vitamin D receptor agonist elocalcitol upregulates L-type calcium channel activity in human and rat bladder. Am J Physiol Cell Physiol 2008;294:C1206-14. [Crossref] [PubMed]
- Curto TM, Giovannucci EL, McKinlay JB, et al. Associations between supplemental or dietary intake of vitamin C and severity of lower urinary tract symptoms. BJU Int 2015;115:134-42. [Crossref] [PubMed]
- Dasgupta J, Elliott RA, Tincello DG. Modification of rat detrusor muscle contraction by ascorbic acid and citric acid involving enhanced neurotransmitter release and Ca2+ influx. Neurourol Urodyn 2009;28:542-8. [Crossref] [PubMed]
- Simaan JA. Herbal medicine, what physicians need to know. J Med Liban 2009;57:215-7. [PubMed]
- Xutian S, Zhang J, Louise W. New exploration and understanding of traditional Chinese medicine. Am J Chin Med 2009;37:411-26. [Crossref] [PubMed]
- Gauruder-Burmester A, Heim S, Patz B, et al. Cucurbita pepo-Rhus aromatica-Humulus lupulus Combination Reduces Overactive Bladder Symptoms in Women - A Noninterventional Study. Planta Med 2019;85:1044-53. [Crossref] [PubMed]
- Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011;186:540-4. [Crossref] [PubMed]
- Huffman MM, Slack A, Hoke M. Bladder Pain Syndrome. Prim Care 2019;46:213-21. [Crossref] [PubMed]
- Parsons M, Toozs-Hobson P. The investigation and management of interstitial cystitis. J Br Menopause Soc 2005;11:132-9. [Crossref] [PubMed]
- Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol 2007;178:145-52. [Crossref] [PubMed]
- Clemens JQ, Brown SO, Kozloff L, et al. Predictors of symptom severity in patients with chronic prostatitis and interstitial cystitis. J Urol 2006;175:963-6; discussion 967. [Crossref] [PubMed]
- Bologna RA, Gomelsky A, Lukban JC, et al. The efficacy of calcium glycerophosphate in the prevention of food-related flares in interstitial cystitis. Urology 2001;57:119-20. [Crossref] [PubMed]
- Parsons CL, Greene RA, Chung M, et al. Abnormal urinary potassium metabolism in patients with interstitial cystitis. J Urol 2005;173:1182-5. [Crossref] [PubMed]
- Bird ET, Parker BD, Kim HS, et al. Caffeine ingestion and lower urinary tract symptoms in healthy volunteers. Neurourol Urodyn 2005;24:611-5. [Crossref] [PubMed]
- Dalsing MC, Grosfeld JL, Remley K, et al. Cyclophosphamide induced hemorrhagic cystitis: the role of protein deficiency in its occurrence. J Pediatr Surg 1982;17:721-27. [Crossref] [PubMed]
- Platzer V, Perez G, Galinier A, et al. Protein and micronutrient deficiencies in patients with radiation cystitis and outcome after hyperbaric oxygen therapy. Clin Nutr ESPEN 2018;23:141-7. [Crossref] [PubMed]
- Farshid AA, Tamaddonfard E, Ranjbar S. Oral administration of vitamin C and histidine attenuate cyclophosphamide-induced hemorrhagic cystitis in rats. Indian J Pharmacol 2013;45:126-9. [Crossref] [PubMed]
- Gurbuz N, Ozkul A, Burgaz S. Effects of vitamin C and N-acetylcysteine against cyclophosphamide-induced genotoxicity in exfoliated bladder cells of mice in vivo. J BUON 2009;14:647-52. [PubMed]
- Farshid AA, Tamaddonfard E, Yahyaee F. Effects of histidine and N-acetylcysteine on diclofenac-induced anti-inflammatory response in acute inflammation in rats. Indian J Exp Biol 2010;48:1136-42. [PubMed]
- Kennedy CM, Bradley CS, Galask RP, et al. Risk factors for painful bladder syndrome in women seeking gynecologic care. Int Urogynecol J Pelvic Floor Dysfunct 2006;17:73-8. [Crossref] [PubMed]
- Weiss JM. Pelvic floor myofascial trigger points: manual therapy for interstitial cystitis and the urgency-frequency syndrome. J Urol 2001;166:2226-31. [Crossref] [PubMed]
- O'Hare PG 3rd, Hoffmann AR, Allen P, et al. Interstitial cystitis patients' use and rating of complementary and alternative medicine therapies. Int Urogynecol J 2013;24:977-82. [Crossref] [PubMed]
Cite this article as: Gefe DB, Thwaini A, Buchholz N. The impact of nutrition and lifestyle on chronic urinary bladder conditions: a literature review. Longhua Chin Med 2024;7:1.

