
Simulation of the auricular branch of the vagus nerve by acupuncture in women with fibromyalgia with severe pain and other functional and emotional problems: study protocol for a randomized, controlled, double-blind clinical trial
Introduction
Approximately 3% of the world population has fibromyalgia (FM) syndrome. Therefore, this problem is the third most common musculoskeletal condition in terms of prevalence, just behind low back pain and osteoarthritis (1,2). FM is characterized by a complex polysymptomatology including widespread chronic pain, fatigue, and unrefreshing sleep, as well as other symptoms not explained by structural or pathologically defined causes (functional symptoms), autonomic disturbances, cognitive dysfunction, somatic symptoms, and psychiatric disorders. The diagnosis of FM is strictly clinical due to the subjectivity of symptoms and the lack of biomarkers (3,4).
FM pathogenesis is not fully understood. The main hypotheses state that there is a genetic predisposition, stressful life events, peripheral (inflammatory) and central (cognitive-emotional) mechanisms that interact to create pain perception due to neuromorphological modifications (“nociplastic pain”) (4,5). Currently, the detection of pro-inflammatory substances and organ-specific and non-specific autoantibodies in the serum of patients with FM has highlighted the role of neuroinflammation in this process. There is evidence of significant differences in peripheral blood cytokine profiles of patients with FM compared to healthy controls, including both pro-inflammatory [such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8] and anti-inflammatory (IL-10, IL-4, IL-5) cytokines, as well as chemokine and other substances (6-8). Therefore, it is significant to investigate treatments that may interfere with the synthesis or release of these substances.
The involvement of many substances, and psychological, behavioral, and social factors make the treatment of FM difficult. The treatment of individuals with FM is complex and spontaneous recovery is unusual. Management aims to improve symptoms, function, and quality of life. The therapy needs to be multimodal and involves aspects of the patient’s education, physical exercises, psychotherapy, and pharmacotherapy (opioids, analgesics, and antidepressants). The effect sizes for non-pharmacological approaches tend to be larger than those for drugs (9). No direct comparisons have shown evidence of the superiority of acupuncture over other therapies to treat FM, despite a growing number of studies supporting the use of acupuncture as part of multimodal treatment approaches with additive efficacy to traditional therapy (10,11). Acupuncture involves stimulating points (acupoints) through the skin, especially with the use of needles. Acupuncture points can be identified on certain parts of the body, including the head, ear, or hand (i.e., scalp, auricular, and hand acupuncture, respectively) (10,11).
There is currently evidence of the use of auricular acupuncture in pain conditions, especially due to the availability of vagal stimulation in the auditory pavilion (12). The vagus nerve originates in the brainstem, runs down both sides of the neck and spreads through the organs of the chest and abdomen, but it also has a branch to the pinna, called the auricular branch of the vagus nerve (ABVN), reaching the center of the ear, where the auricular concha and the auditory canal are located (13). Preclinical studies have shown that vagal stimulation effectively modulates pain, and the use of stimulation devices has been investigated in humans (14). Furthermore, studies from our laboratory show that there seems to be a difference between the effects observed in the stimulation of the right and left ABVN in rats (15). The treatment with ABVN stimulation in FM individuals did not give additional benefit together with exercise, except for three subparameters of functional evaluation—Short Form-36 (SF-36). However, there is a need for further studies that separately investigate the effects of ABVN stimulation and other therapies in FM, with longer follow-up and a larger number of patients, in addition to more effective blinding (16).
The primary purpose of this study is to evaluate the effect of ABVN stimulation by acupuncture in individuals with FM while observing pain indices and comparing the treatment performed on the left and right ears. Furthermore, we will analyze associated problems such as depression, anxiety, catastrophic thinking, sleep quality, and functionality. Additionally, we will conduct a pre-planned secondary analysis to determine the blood concentration of inflammatory mediators, as well as anti-inflammatory cytokines, in women with FM. Finally, we will correlate the primary outcome, pain, with the secondary outcomes of depression, anxiety, catastrophic thinking, sleep quality, functionality, and the concentration of inflammatory and anti-inflammatory mediators. We present this article in accordance with the SPIRIT and TIDieR reporting checklists (available at https://lcm.amegroups.com/article/view/10.21037/lcm-23-8/rc).
Methods
Trial design
The study is a double-blind, randomized controlled clinical trial (patient and evaluator) and will evaluate the effects of acupuncture ABVN stimulation in individuals with FM and compare treatment performed in left and right ear. Figure 1 outlines the trial phases.
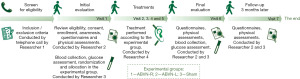
Participants and eligibility criteria
In this study, 51 adult women (randomized into three groups) will be recruited. The study eligibility criteria are women aged between 18–65 years; with the diagnosis of FM, according to the American College of Rheumatology (ACR) criteria. The participant will be evaluated and screened for eligibility under the supervision of the first researcher, a neurologist doctor, via telephone contact (Figure 1). Researcher 1 will take the initial data, verify the participant’s eligibility, and will apply the consent term. Participants will be ineligible (exclusion criteria) for participation if they have cancer; suspected or confirmed pregnancy; cognitive problems that compromise assessments or treatment, such as misunderstanding of questionnaires; unstable heart conditions; lesions in the ear pinna, or absence thereof.
Recruitment, randomization, and allocation
This research will be carried out in the Ambulatory rooms of the Department of Physiological Sciences, under the care of the Pain and Inflammation Neurobiology Laboratory, located at the Biological Sciences Center of the Federal University of Santa Catarina, in Florianópolis, Santa Catarina, Brazil. Recruitment will be carried out in the city of Florianópolis (Santa Catarina, Brazil), through advertisements in the community and social networks. An anamnesis, questionnaires, physical assessments (Researcher 2), blood collection, and glucose assessment (Researcher 3) will be performed during the first visit.
The randomization process will be previously generated using Excel 2010 software (Microsoft), and the allocation in the experimental group will be performed after the procedure by the same Researcher 3 (Figure 1). Each experimental group will receive a corresponding color [e.g., blue—ABVN-right (ABVN-R) stimulation; yellow—ABVN-left (ABVN-L) stimulation; green—Sham treatment] and papers with this description will be placed inside an opaque sealed envelope. Researcher 3 will draw the envelope, keeping it in the participant’s folder. The envelope will be opened only on the first treatment session by Researcher 4, who is responsible for the treatments of the individuals. The envelope will be stored in a place accessed only by the team participating in the treatments.
The research team and blinding
The entire research team was previously trained, with the completion of a training course to qualify the team in the techniques and to standardize the assessments and treatments of this study. The course was conducted by researchers Neves ML and da Silva MD, physical therapists (PT), and acupuncturists with years of experience in the topics covered. The team comprises undergraduate and graduate students from the Federal University of Santa Catarina (UFSC), as well as volunteers (health professionals interested in scientific research).
Relevant information: (I) first, make contact with participants and perform the initial eligibility assessment; (II) each researcher is part of only one group, being an evaluator or a therapist; (III) all evaluators (Researchers 2 and 3) are blinded to which experimental group the participant belongs to. They will apply the scale or questionnaire, without influencing the patients’ responses, only answering questions, if any; (IV) the therapy will be under the responsibility of a physiotherapist with more than 20 years of experience in acupuncture (Researcher 4). The therapists are not part of the assessment team; (V) data tabulation and statistical analysis will be performed respectively by two individuals blinded to the experimental groups, where only they will have access to this data.
Intervention
Patients screened and examined will be allocated to experimental groups: 1—ABVN-R group; 2—ABVN-L group; or 3—Sham group. The therapists will treat the participants according to the proposed groups, and the intervention will be carried out once a week for four consecutive weeks. All participants will be directed to the treatment room (two rooms without mirrors or reflective objects were reserved), instructed to sit comfortably in a chair, next to a stretcher, and close to the needle disposal site. Participants are advised not to use their cell phones during treatment, and the appointment schedule is programmed so that participants do not meet in the corridors of the building, avoiding conversations between them.
ABVN-R group: ABVN stimulation will be performed using two Qi Zhou acupuncture needles (0.20 mm × 15 mm). The needles will be inserted using an applicator to a depth of 2–3 mm from the surface of the auricular skin in the superior (center of the conch crest) and inferior (center of the conch cava) regions of the concha of the right pavilion (ABVN-R). They will be stimulated with manual half-turns in both directions—once per second, first to the right, then to the left, and so on for 30 seconds. At the end of the stimulation, the needles will be maintained for 10 minutes and then removed and discarded in front of the participant, prompting him to view the discard. Next, on the same two stimulated points, two vaccaria seed spheres will be placed, and fixed with microporous tape (Micropore—3M). Patients will be instructed to stimulate each point for 10 seconds, three times daily (once per day shift). On the fourth day, the seeds must be removed by the patient. If they fall off spontaneously, the patient will be advised not to replace the auricular points. On the following week (day seventh after ABVN stimulation), participants should return for the next session, when the same procedure will be repeated.
ABVN-L group: the entire treatment procedure will be similar to the one described in the ABVN-R group; however, the stimulated auricular points will be in the left ear pavilion (L).
Sham group: simulated treatment will be performed. For this, the therapist will use the applicator in the upper (center of the conch crest) and inferior (center of the conch cava) regions of the shell of the left pavilion (ABVN-L), but without the application of needles. Instead, a metal rod with a blunt tip and 1 mm in diameter will be used (17,18). The manual half-turns of the needles will be simulated, in the same way described above, for 30 seconds. At the end of the stimulation, the patient remains still for 10 minutes, and then the needle removal will be simulated. The therapist discreetly places a needle in her hand, places it close to the participant’s ear, and then discards the object in the appropriate location, requesting the patient to visualize the discard. Next, on the same two stimulated points, two vaccaria seed husks will be fixed with microporous tape (Micropore—3M). Patients will be instructed to keep the patches on until the next session. On the following week (day seventh after ABVN stimulation or placebo stimulation), participants should return for the next session, when the same procedure is repeated.
Outcome measures
Primary outcomes
Individuals with FM have widespread pain and increased sensitivity to mechanical pressure and cold temperatures. Therefore, the primary outcome will be the pain assessed by a Numerical Pain Rating Scale (NPRS) (4,19).
Secondary outcomes
Other forms of assessing pain will be used as secondary outcome measures, such as algometry and generalized pain index questionnaires, FM impact questionnaire, and catastrophic thoughts about pain scale. In addition, FM is also characterized by the presence of several other symptoms, especially fatigue, and unrefreshing sleep, so measures to assess sleep, quality of life, fatigue, and function will be used, such as symptoms severity with patient-specific functional scale and dynamometry. Moreover, autonomic system assessments and general health measures will be performed, such as checking blood pressure, heart rate variability (HRV), oxygen saturation, and glucose.
Importantly, the presence of psychiatric disorders will be verified with scales to assess depression and anxiety. Stimulation of the ABVN-R, but not the left branch, is linked to dopaminergic neurons in the brainstem, an essential neural circuit for motivation and reward (20), so there may be a difference in the stimulation of the ABVN-R and ABVN-L. In this way, the observation of the effect and the difference between the data of these experimental groups (ABVN-R versus ABVN-L stimulation) will also be verified in the present research. Although there are no specific biochemical markers for the disease, measures of inflammatory and anti-inflammatory mediators, such as cytokines tumor necrosis factor (TNF)-α, interleukin IL-6, IL-8 and anti-inflammatory IL-10, IL-4, IL-5, will be analyzed. Data related to research outcomes will be collected by blinded evaluators at baseline, in four weeks, and three months after the first session, for all outcomes except blood analysis, which will be evaluated only at baseline and after four weeks of treatment. The importance of full follow-up will be emphasized and accompanied by the main researcher, and if any patient interrupts the treatment, she will be contacted by telephone to find out the reason. In case of withdrawal, her data will later be analyzed as an intention to treat.
Assessment of pain
The NPRS is a unidirectional pain assessment instrument used in adults. It is a numerical version of the Visual Analogue Scale, in which an individual chooses the number (minimum of 0 and maximum of 10 points) that best describes the intensity of their pain (0—no pain and 10—worst pain ever) (21).
The Generalized Pain Index is calculated as the sum of 19 body areas referred to as painful by patients, with one point for each area.
Meanwhile, the Symptom Intensity Scale is composed of an assessment of symptoms such as fatigue, non-restorative sleep, cognitive disorders, and somatic disorders on a severity scale (22).
Fibromyalgia Impact Questionnaire (FIQ) is the instrument that specifically assesses the quality of life in patients with FM, involving issues related to functional capacity, professional status, psychological disorders, and physical symptoms, including pain. The higher score represents a greater impact of FM on quality of life, with the maximum score being 100 (23).
Finally, the Catastrophic Thoughts About Pain Scale is composed of two scales (coping and catastrophizing strategies) associated with the words almost never and almost always at their ends. Its internal correlation coefficient (Cronbach α) is 0.89, which suggests that the internal consistency of this scale is adequate (24).
Other evaluation related to FM
The Beck Depression Inventory (BDI) is a self-rated measure of depression that consists of 21 items, including symptoms and attitudes. The items refer to sadness, pessimism, feelings of failure, lack of satisfaction, feelings of guilt, feelings of punishment, self-depreciation, self-accusations, suicidal thoughts, crying spells, irritability, social withdrawal, indecision, distortion of body image, work inhibition, sleep disturbance, fatigue, loss of appetite, weight loss, somatic worry, decreased libido. The internal consistency of the scale is 0.81 for females (25).
The Beck Anxiety Inventory (BAI) consists of twenty-one questions about how the individual has been feeling in the last week, expressed in common anxiety symptoms such as sweating and feelings of distress. Each item has four possible answers, respectively: no, slightly, moderately, and severely. The Brazilian version showed good psychometric characteristics, with a Cronbach’s α of 0.88 (26).
The Pittsburgh Sleep Quality Index (PSQI) is a questionnaire that assesses sleep quality and its disturbances over a period of 1 month prior to its application. It was developed by Buysse and collaborators (27) and validated for Portuguese in 2011 by Bertolazi et al. (28). The global score ranges from 0 to 21, and the higher the score, the worse the sleep quality (27).
The Specific Function Scale is an instrument where the patient reports the three items, she considers herself most unable to perform in her activities of daily living. In addition to reporting the activities, the patient gives a score from 0 to 10 regarding her level of capacity for this activity, where 0 is totally incapable and 10 is totally capable (29).
HRV is considered the gold standard for quantitative autonomic balance, providing an indirect view of autonomic tone. It may also be an important marker of psychological well-being (30). High HRV is related to good adaptive capacity in different situations, or stimuli, just as low HRV can be associated with greater health risk (31). Anxiety disorders are associated with reduced HRV, just as low HRV is associated with low parasympathetic activity (32). The HRV assessment will be performed during the first and fourth consultations, with the patient sitting, using the Polar H10 equipment and the Elite HRV application, doing continuous recording for 20 minutes, 5 minutes at baseline before acupuncture, and 5 minutes after placing the needles or vaccaria seeds. The extracted data will be analyzed by the Kubios program.
Pressure pain threshold will be assessed using an algometer (JTECH Commander, Salt Lake City, USA). The pressure applied to the skin of the individuals will be performed three times at each point. These points feel the belly of the superior trapezius and tibialis anterior muscles. Pressure will be measured in pounds; peak pressure will be recorded automatically, and the average of three repetitions will be used for statistical analysis (33).
The evaluation of the strength of the upper limbs, through handgrip, will be performed using a digital dynamometer (Instrutherm model DM-90) with a unit of measurement of a kilogram (kg) and a maximum capacity of 90 kg and accuracy of +/−0.5 kg. The patient will be sitting with the upper limb at 90 degrees and without assistance in supporting the instrument. The test will be performed three times on each limb, in a non-alternating manner, with a verbal encouragement command by the evaluator, holding for 3 seconds in maximum grip and an interval of 30 seconds between each test. The largest measure will be taken as a reference value on each side (34).
Blood pressure will be checked using an automatic digital arm sphygmomanometer (Omron HEM-7122, São Paulo, Brazil), while blood oxygen saturation will require using a finger oximeter (G-Tech Portable Oled Graph, Barueri, São Paulo, Brazil), and the blood glucose level will be verified through a capillary blood glucose test (ACCU CHEK ACTIVE—ROCHE, São Paulo, Brazil).
Biomarker selection and measurement: venous blood samples will be collected in test tubes containing a separation gel and centrifuged at 7,000 rpm for 10 min (4 ℃). The supernatant will be collected and stored in a −80 ℃ freezer until analysis. Cytokines kits will be used to measure the serum levels of cytokines by enzyme-linked immunosorbent assay (ELISA, kits from R&D Systems, Minneapolis, USA). Will be used 100 µL of sample aliquots for each measurement of the following cytokines in the present work: IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, TNF-α, and interferon (IFN)-γ. Sample processing will be performed according to the manufacturer’s specifications. The absorbance for all cytokines studied will be performed using a plate reader of 450 and 570 nm (multi-reader infinite M200 TECAN—LAMEB/UFSC). The investigators performing the assays will be blinded to the patient group.
Data analysis
The sample size was calculated using the online program http://estatistica.bauru.usp.br/calculoamostral/ta_comparacao_multipla_independentes.php. The calculation was performed by estimating a minimum number necessary for a pilot study, as well as a minimum detectable difference of 2 points on the numeric pain scale at a 12-week follow-up, an estimated standard deviation of 1.6, and a significance level of 0.05, and a test power of 80% (35). This calculation generated a sample of 14 individuals per group, totaling 42 subjects. Assuming a conservative estimate of 20% dropout from the study, the recruitment of 17 individuals per group was planned, totaling 51 subjects.
Descriptive data and scores for the primary and secondary outcomes will be tabulated in Microsoft Excel 2010. The statistical package for the social sciences, SPSS—Statistical Package for the Social Sciences, version 15.0 (IBM Corp., Armonk, NY, USA) or GraphPad Prism, version 8.0 (GraphPad Software, Inc., San Diego, CA, USA) will be used for data analysis. The significance level will be 0.05 for all analyses. Data with sample characteristics will be expressed as absolute and relative frequencies, using mean and standard deviation (SD) for quantitative variables and with normal distribution in the Shapiro-Wilk test, or using median and interquartile range (IQR). In the presentation of categorical variables, the chi-square test will be used. To compare the quantitative variables of the groups, all data must pass the Shapiro-Wilk normality test, Mauchly’s sphericity test, and Levene’s homogeneity test. For normal data, mixed factorial ANOVA will be used, followed by Bonferroni’s post hoc; for data that do not have homogeneous variances or normal distribution, the non-parametric Mann-Whitney U test will be used.
Monitoring
In this study, data will be managed by a blinded data entry and management team. The training course was carried out prior to the recruitment of participants, but the research team will hold periodic meetings every month, to review the safety, verify experiences and modify where appropriate. No formal audit is scheduled for this trial. Human subject approval will be maintained using a central system at the institution, and all needed amendments will be documented in the manual of procedures with corresponding updates to relevant parties.
Research ethics approval & clinical trial registration
The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was registered in the Brazilian Registry of Clinical Trials (RBR-10d3crcf) after its approval by the Research Ethics Committee of the Federal University of Santa Catarina (CAAE 36783520.9.0000.0121). We have fewer than 50% of participants enrolled, and all of them will provide informed consent prior to participation in the trial, signing the Free and Informed Consent Form (ICF) before participating in the study. The checklist protocols were sent for review by the journal: (I) Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement; (II) Template for Intervention Description and Replication (TIDieR) checklist.
Trial results
Data collection is expected to be completed by early 2023. Data analysis and manuscript preparation are expected to be completed in mid-2023. The research team intends to perform data analysis to publish the results in a peer-reviewed journal and present findings at professional conferences. Possible harm and adverse effects reported by patients will also be described in the results.
Discussion
This is the first large-scale study designed to assess the effect of ABVN stimulation by acupuncture in individuals with FM. This study has the potential to provide information on auricular treatment and can substantially impact the field of rehabilitation, especially in patients with chronic pain, such as individuals with FM. Importantly, especially for countries with a broad health system such as Brazil, the data from the present research may provide evidence for early intervention programs, which may include complementary therapies such as ABVN stimulation in the treatment of chronic diseases.
Unfortunately, a limitation of the study is the impossibility of blinding the therapists. Furthermore, this study is not going to be conducted multicentrically and will have only women in the sample, which may narrow our results down to just one population. In addition to the data on the therapy studied, the data from the present research can also provide evidence of biological markers found in individuals with FM, serving as input for the diagnosis of the disease or its intensity.
Our results can directly impact health and rehabilitation professionals, policymakers, patients and their families. Future studies should be carried out with a larger sample size and longer treatment time to further validate the therapeutic effects of acupuncture on ABVN in patients with FM.
Acknowledgments
We are immensely grateful to Prof. Dr. Adair R. S. dos Santos (Federal University of Santa Catarina), a great friend, and instructor, who contributed intensely to our work (providing concept/idea/research design/facilities) and recently passed away, being deeply missed. We also thank Neuroscience Post-Graduate Program at the Federal University of Santa Catarina (UFSC) and Multiuser Laboratory of Biology Studies (LAMEB/UFSC), as well as Programa INCT-INOVAMED (No. 465430/2014-7) grant.
Funding: The project was funded by the Foundation for Research Support and Innovation of the State of Santa Catarina (FAPESC), through the Public Call Notice Fapesc nº 16/2020 – Research Program for SUS: shared management in health.
Footnote
Reporting Checklist: The authors have completed the SPIRIT and TIDieR reporting checklists. Available at https://lcm.amegroups.com/article/view/10.21037/lcm-23-8/rc
Trial Protocol: Available at https://lcm.amegroups.com/article/view/10.21037/lcm-23-8/tp
Peer Review File: Available at https://lcm.amegroups.com/article/view/10.21037/lcm-23-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://lcm.amegroups.com/article/view/10.21037/lcm-23-8/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was registered in the Brazilian Registry of Clinical Trials (RBR-10d3crcf) after its approval by the Research Ethics Committee of the Federal University of Santa Catarina (CAAE 36783520.9.0000.0121). We have fewer than 50% of participants enrolled, and all of them will provide informed consent prior to participation in the trial, signing the Free and Informed Consent Form (ICF) before participating in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spaeth M. Epidemiology, costs, and the economic burden of fibromyalgia. Arthritis Res Ther 2009;11:117. [Crossref] [PubMed]
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep 2013;17:356. [Crossref] [PubMed]
- Talotta R, Bazzichi L, Di Franco M, et al. One year in review 2017: fibromyalgia. Clin Exp Rheumatol 2017;35:6-12. [PubMed]
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol 2020;16:645-60. [Crossref] [PubMed]
- Häuser W, Sarzi-Puttini P, Fitzcharles MA. Fibromyalgia syndrome: under-, over- and misdiagnosis. Clin Exp Rheumatol 2019;37:90-7. [PubMed]
- Bazzichi L, Rossi A, Giuliano T, et al. Association between thyroid autoimmunity and fibromyalgic disease severity. Clin Rheumatol 2007;26:2115-20. [Crossref] [PubMed]
- Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med 2010;16:1267-76. [Crossref] [PubMed]
- O'Mahony LF, Srivastava A, Mehta P, et al. Is fibromyalgia associated with a unique cytokine profile? A systematic review and meta-analysis. Rheumatology (Oxford) 2021;60:2602-14. [Crossref] [PubMed]
- Kwiatek R. Treatment of fibromyalgia. Aust Prescr 2017;40:179-83. [Crossref] [PubMed]
- Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev 2013;2013:CD007070. [Crossref] [PubMed]
- Berger AA, Liu Y, Nguyen J, et al. Efficacy of acupuncture in the treatment of fibromyalgia. Orthop Rev (Pavia) 2021;13:25085. [Crossref] [PubMed]
- Frøkjaer JB, Bergmann S, Brock C, et al. Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. Neurogastroenterol Motil 2016;28:592-8. [Crossref] [PubMed]
- Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458-62. [Crossref] [PubMed]
- Shao P, Li H, Jiang J, et al. Role of vagus nerve stimulation in the treatment of chronic pain. Neuroimmunomodulation 2023; Epub ahead of print. [Crossref] [PubMed]
- Neves ML, Karvat J, Simões RR, et al. The antinociceptive effect of manual acupuncture in the auricular branch of the vagus nerve in visceral and somatic acute pain models and its laterality dependence. Life Sci 2022;309:121000. [Crossref] [PubMed]
- Kutlu N, Özden AV, Alptekin HK, et al. The Impact of Auricular Vagus Nerve Stimulation on Pain and Life Quality in Patients with Fibromyalgia Syndrome. Biomed Res Int 2020;2020:8656218. [Crossref] [PubMed]
- Vas J, Modesto M, Aguilar I, et al. Effects of acupuncture on patients with fibromyalgia: study protocol of a multicentre randomized controlled trial. Trials 2011;12:59. [Crossref] [PubMed]
- Sherman KJ, Hogeboom CJ, Cherkin DC, et al. Description and validation of a noninvasive placebo acupuncture procedure. J Altern Complement Med 2002;8:11-9. [Crossref] [PubMed]
- Goebel A, Krock E, Gentry C, et al. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest 2021;131:e144201. [Crossref] [PubMed]
- Han W, Tellez LA, Perkins MH, et al. A Neural Circuit for Gut-Induced Reward. Cell 2018;175:665-678.e23. [Crossref] [PubMed]
- Misailidou V, Malliou P, Beneka A, et al. Assessment of patients with neck pain: a review of definitions, selection criteria, and measurement tools. J Chiropr Med 2010;9:49-59. [Crossref] [PubMed]
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600-10. [Crossref] [PubMed]
- Marques AP, Santos AMB, Assumpção A, et al. Validation of the Brazilian Version of the Fibromyalgia Impact Questionnaire (FIQ). Braz Journ of Rheumatol 2006;46:24-31.
- Junior JS, Nicholas MK, Pereira IA, et al. Validation of the Pain-Related Catastrophizing Thoughts Scale. Acta Fisiatr 2008;15:31-6.
- Andrade L, Gorenstein C, Vieira Filho AH, et al. Psychometric properties of the Portuguese version of the State-Trait Anxiety Inventory applied to college students: factor analysis and relation to the Beck Depression Inventory. Braz J Med Biol Res 2001;34:367-74. [Crossref] [PubMed]
- Quintão S, Delgado AR, Prieto G. Validity study of the Beck Anxiety Inventory (Portuguese version) by the Rasch Rating Scale model. Psicol Reflex Crit 2013;26:305. [Crossref]
- Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193-213. [Crossref] [PubMed]
- Bertolazi AN, Fagondes SC, Hoff LS, et al. Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med 2011;12:70-5. [Crossref] [PubMed]
- Costa LO, Maher CG, Latimer J, et al. Clinimetric testing of three self-report outcome measures for low back pain patients in Brazil: which one is the best? Spine (Phila Pa 1976) 2008;33:2459-63. [Crossref] [PubMed]
- Vanderlei LC, Pastre CM, Hoshi RA, et al. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc 2009;24:205-17. [Crossref] [PubMed]
- Ernst G. Heart-Rate Variability-More than Heart Beats? Front Public Health 2017;5:240. [Crossref] [PubMed]
- Chalmers JA, Quintana DS, Abbott MJ, et al. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry 2014;5:80. [Crossref] [PubMed]
- Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain 1987;30:115-26. [Crossref] [PubMed]
- Reis MM, Arantes PMM. Assessment of hand grip strength - validity and reliability of the saehan dynamometer. Fisioterapia e Pesquisa, São Paulo 2011;18:176-81. [Crossref]
- Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287-91. [Crossref]
Cite this article as: Neves ML, Ferreira JB, Kraus SI, Speretta GFF, Amaral PAH, Wippel VA, Gentil LB, Rodrigues IK, da Silva MD. Simulation of the auricular branch of the vagus nerve by acupuncture in women with fibromyalgia with severe pain and other functional and emotional problems: study protocol for a randomized, controlled, double-blind clinical trial. Longhua Chin Med 2023;6:5.

