
Integrative medicine management of median arcuate ligament syndrome: a case report
Highlight box
Key findings
• Acupuncture may provide adjunctive management and pain relief for patients with Median Arcuate Ligament Syndrome (MALS)
• Acupuncture points to support Yin deficiency and address Qi stagnation were used and may improve symptoms in certain cases
What is known and what is new?
• MALS is characterized by compression of the celiac artery and nerve plexus due to anatomical variation of the median arcuate ligament
• MALS is difficult to diagnose and treat due to its broad abdominal symptoms and difficult identification on traditional imaging modalities
• Non-surgical treatment strategies for MALS are limited; acupuncture may provide symptomatic relief in refractory cases or when surgery is not readily available
What is the implication, and what should change now?
• Physicians should consider MRA imaging for MALS in patients with non-specific abdominal pain and an otherwise negative work-up
• If MALS is diagnosed, utilization of acupuncture by a trained practitioner may improve both abdominal symptoms and stress in patients with MALS, especially in the perioperative period
Introduction
Median arcuate ligament syndrome (MALS) is a condition characterized by an anatomically inferior median arcuate ligament (MAL) resulting in impingement on the celiac trunk and/or celiac ganglia. Compression of the celiac trunk is thought to cause foregut ischemia with hallmark symptoms including post-prandial or exercise-induced epigastric pain, early satiety, nausea, or vomiting, with food aversion and weight loss. Further, nearby celiac ganglia, which carry sympathetic nerve fibers and receive visceral pain signals from the foregut may also be implicated, leading to radiating discomfort, epigastric fullness and delayed gastric emptying (1). Studies have shown MALS is about 4 times more common in females and often diagnosed in patients between 30 and 50 years old (2). On physical exam, patients may have severe epigastric tenderness, marked weight loss, and abdominal bruits which are louder with expiration (2). Current treatments include surgical interventions such as placement of a stent or reconstruction of the celiac artery, laparoscopic MAL release, and/or celiac ganglionectomy (3). Research on supportive measures for MAL-related symptoms is limited. Here we present a case of MALS that uniquely improved with acupuncture and trigger point injections prior to surgical intervention, a finding not previously reported in the literature.
We present the following case in accordance with the CARE reporting checklist (available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-24/rc).
Case presentation
A 44-year-old female with history of irritable bowel syndrome (IBS), rheumatoid arthritis (RA), seasonal allergies, lumbar spinal stenosis, and white coat hypertension presented to the emergency department with severe epigastric pain. Weight at initial admission was 125 lb, with an approximate 13 lb weight loss in one month. The patient had a prior history of abdominal pain with resultant appendectomy and cholecystectomy and was admitted for further work-up. Her abdominal pain began following a 5-day supported fast of 500 kcal/day. Her symptoms intensified over a month with persistent mid- and epigastric abdominal pain, rated 5/10 at baseline, with occasional radiation to her chest. Her pain intensified to the point of being unable to tolerate food or liquids except for water. Drawing her legs up to her chest alleviated the pain. Additionally, she reported an inability to lie flat and associated intermittent diarrhea. She reported no nausea, vomiting, or constipation.
General surgery was consulted for surgical management of MALS. It was discussed that surgical release was not an urgent intervention and plans to follow-up with the patient at discharge were initiated. Further, due to her inability to tolerate solid foods with a significant weight loss, general surgery recommended parenteral nutrition to regain strength and prevent malnourishment. Due to the temporizing nature of this intervention, the patient was initiated on total parenteral nutrition (TPN) for approximately 8 weeks. Her opioid regimen included morphine extended release 15 mg three times per day and morphine immediate release 15 mg every 4 hours. Non-opioid analgesic regimen included methocarbamol 1,000 mg four times per day, gabapentin 600 mg three times per day and acetaminophen. She was also started on ondansetron for nausea, pantoprazole for prophylaxis, simethicone, and sucralfate.
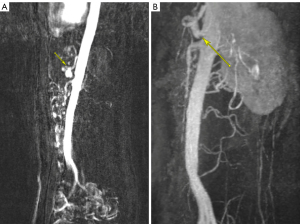
Prior to admission, the patient had undergone an abdominal ultrasound which visualized a normal proximal aorta and inferior vena cava. She also had a transvaginal and transabdominal ultrasound with normal doppler flow to both ovaries and an 18 mm subserosal fundal fibroid identified. An upper endoscopy demonstrated Los Angeles Grade A esophagitis with a normal duodenum and biopsies negative for Heliobacter pylori and celiac disease. Lastly, a CT abdomen/pelvis identified a 17 mm nodular density in the gastric antrum otherwise did not demonstrate abnormalities (see Figure 1). Due to the previously negative studies, the patient underwent magnetic resonance angiography (MRA) of the abdomen and pelvis. Findings demonstrated narrowing of the celiac artery to 2 mm with a J-shaped appearance at the level of the MAL with post-stenotic dilatation up to 9 mm compatible with MALS (see Figure 2A). Despite an intensive pharmacologic pain regimen, the patient continued to have severe gastrointestinal symptoms and discomfort. Ultimately, surgical intervention was planned as the curative option. However, while awaiting surgery, East-West Medicine was consulted for non-pharmacologic pain control. The East-West consult service is a unique inpatient service including physicians trained in traditional Chinese medicine (TCM) modalities including acupuncture as well as trigger point injections for adjunctive pain management.
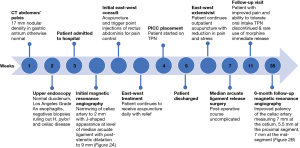
On exam, the patient’s pulse was wiry at the left middle positions and tight at the right middle position. Tongue body was pink with darker sides with a thin white tongue coat. Abdomen was tender to palpation in the epigastrium without rebound tenderness or involuntary guarding. From a TCM perspective, the patient was diagnosed with Rebellious Stomach Qi due to Liver Qi Stagnation. She was provided with acupuncture for adjunctive pain control.
Acupuncture was performed once daily during six individual sessions. Each session was performed by one physician who is board-certified in Internal Medicine and had completed UCLA’s East-West fellowship which provided 2 years of robust training in acupuncture and TCM. Each acupuncture session lasted approximately 30 minutes. Points utilized include Large Intestine (LI) 4; Liver (LV) 3; Stomach (ST) 23–25, 36; Spleen (SP) 6, 10; Conception Vessel (REN) 6, 12, 13; Kidney (KI) 3; and Gallbladder (GB) 34. Auricular acupuncture was conducted in the left ear at shen men, sympathetic point, and point zero. These acupuncture points were chosen to support her Yin deficiency (KI 3), improve gastric motility and abdominal pain (ST 23–25, ST 36). LI 4 and LV 3 were selected to reduce pain and induce calm. LV 3 and GB 34 were chosen to promote Qi flow and reduce Qi stagnation. ST 23–25, REN 12, REN 13, and REN 6 are local points for abdominal pain (see Figure 3). There were no side effects associated with the acupuncture sessions.

She also received trigger point injections twice with 1% lidocaine to the lateral regions of the rectus abdominal muscles bilaterally to assist in myofascial strain, involuntary upper abdominal guarding, and improved diaphragmatic relaxation.
She specifically noted subjective improvement in epigastric pain, lying flat, and an increased sense of relaxation facilitating discharge from the hospital.
While awaiting surgery, the patient continued to seek once to twice weekly East-West Medicine outpatient visits for symptomatic control including acupuncture with another physician board-certified in Internal Medicine and a graduate of UCLA’s East-West fellowship. Similar to inpatient, acupuncture sessions lasted approximately 30 minutes. The patient continued to have improvement in abdominal pain and stress management with acupuncture treatments.
Three weeks following hospital discharge the patient underwent exploratory laparotomy with surgical release of the MAL with an uncomplicated post-operative course. At 1-month postoperative follow-up, TPN was discontinued, and the patient reported improved pain with cessation of morphine extended release and rare use of morphine immediate release. Follow-up MR abdomen angiogram with contrast was done approximately 6 months post-operatively with significant improvement in the patency of the celiac axis measuring 7 mm at the ostium, 5.5 mm through the proximal segment and 7 mm at the mid-segment (see Figure 2B).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal
Discussion
MALS is a chronic condition due to compression of the celiac trunk and nearby celiac ganglia due to an inferiorly placed MAL. The MAL is a fibrous arch connecting the left and right crura of the diaphragm by traversing anteriorly to the aorta and normally superior to the celiac trunk at the level of the aortic hiatus (T12-L1).
While the exact prevalence of MALS is unknown, several studies have been undertaken to quantify the percentage of the population affected. Two retrospective studies of patients undergoing computed tomography angiography (CTA) demonstrated evidence of celiac artery compression by the MAL in 2.8% and 3.4% of cases, respectively [N=744 (4); N=344 (5)]. A study on autopsy specimens found that 1/3 of subjects had significant anatomic variability with the MAL more inferiorly placed (1). Long-term compression may cause hyperplastic intimal changes of the celiac artery causing arterial occlusion with poststenotic dilation and aneurysm development (2). Another study on cadaveric subjects demonstrated that 37% of subjects with MALS had kinking of the celiac trunk, as was also seen in this patient case (6). Interestingly, a significant proportion of patients with MALS develop collateral circulation, with one study demonstrating a significantly positive correlation between the degree of celiac artery stenosis and collateral arterial supply (r=0.339; P<0.001) (7).
Further, the celiac ganglion, a nerve plexus supplying the foregut and located laterally to the celiac trunk may be implicated in neuropathic pain experienced by MALS patients. One study performed a temporary percutaneous celiac plexus block using a local anesthetic agent in 4 patients with 100% describing symptom relief after the block (8).
MALS may be difficult to diagnose as there is no currently accepted diagnostic criteria and some patients with evidence of celiac trunk compression by the MAL may be asymptomatic. However, researchers have tried to delineate a diagnostic algorithm for patients with persistent epigastric pain despite an otherwise negative work-up. Abdominal duplex ultrasonography (DUS) is considered first-line imaging as it permits better appreciation in changes in vessel caliber and flow velocity, while costing less and avoiding radiation exposure. In a retrospective cohort study of patients undergoing DUS of the celiac trunk, 100% of patients diagnosed with MALS had celiac trunk deflection angles >50 degrees compared with 40% of patients in the asymptomatic control group (N=6 vs. N=20) (9). There was also a statistically significant decrease in expiratory peak systolic flow velocities between the MALS patients and the asymptomatic controls (P=0.001) (9). CTA and/or MRA imaging allow visualization of celiac artery narrowing as well as the classic hooked appearance—both modalities should ideally be performed with inspiration and expiration to observe changes in celiac artery compression (2).
While definitive treatment includes decompression of the MAL (e.g., via robotic, laparoscopic, endoscopic retroperitoneal or open surgical intervention) with the possibility for celiac ganglionectomy, TCM may offer a robust non-pharmacologic approach to pain control.
Acupuncture is a practice that was developed in China over 3,000 years ago. It involves placing thin needles into specific points along the body to help realign one’s “qi” or energy flow (10). There is evidence that acupuncture increases the release of opiate-like peptides in the brain to provide pain relief (10). Previous research has elucidated that acupuncture may activate Type A-delta (localized cutaneous pain signals) and Type C (visceral pain signals) nerve fibers to increase endogenous opioid production and downregulate transmission of pain (10).
Acupuncture is significantly better than sham treatment at improving gastroparesis symptoms, both with short- and long-term efficacy (11,12). In a Cochrane review of gastroparesis, short-term treatment with acupuncture was superior to symptomatic management with gastrokinetic agents, although the data is limited, and long-term studies have not been conducted (13). Acupuncture may increase gastric motility in patients with low baseline peristalsis yet can paradoxically suppress peristalsis in patients with high initial gastric motility (10,14).
Specifically, Zusnali (ST36) has been shown to increase gastric motility by increasing vagal parasympathetic tone and release of oxytocin (14). ST36 has been shown to normalize contractions of the gastric antrum through upregulation of interstitial cells of Cajal, the pacemaker cells of peristalsis in the gut (14). Prior research utilizing electroacupuncture at ST36 also demonstrated increase in motilin and cholecystokinin, two potent proteins that increase gut motility (14). Because MALS is linked with foregut ischemia and impaired gastric motility due to celiac artery compression, ST36 is a uniquely apt point and was thus utilized in all 6 acupuncture sessions with our patient.
Hegu (LI4) is another acupuncture point that was used in all 6 TCM sessions with our patient—prior research has demonstrated that LI4 may inhibit excessive GI motility thus helping to reset an overactive digestive tract and thus may explain the improved epigastric pain and motility in our patient after each session (14).
Tai chong (LV3) is an acupuncture point on the dorsum of the foot in between the 1st and 2nd metatarsal bones and has also been shown to be helpful for managing nausea, vomiting, and abdominal pain (15).
One study of patients undergoing acupuncture of LV3, LI4, and ST36 during functional MRI demonstrated modulated brain activity; specifically, activation of the anterior insular and prefrontal areas (pain-associated brain networks) persisted after treatment and may posit a possible mechanism of the analgesic effect of these acupuncture points (16).
Tianshu (ST25) is an abdominal acupuncture point that was used in 3 sessions. In a rat model of IBS, electroacupuncture of ST25 demonstrated improved visceral hypersensitivity, decreased withdrawal from abdominal pressure/stimulation, as well as decreased levels of serotonin (5-HT) which may be dysregulated in motility disorders (17).
We presented a case of radiologically diagnosed MALS managed with acupuncture and other integrative medicine modalities (e.g., trigger point injections) resulting in significant improvement in pain and stress reduction. While MALS is a diagnosis of exclusion, the anatomic MAL variant may be quite prevalent in the population, unknowingly contributing to post-prandial epigastric pain, nausea, vomiting, and weight loss. Unfortunately, many chronic abdominal pain patients with an otherwise negative work-up may be prescribed opioids, which are linked to delayed gastric emptying. As gastric motility is exacerbated by MALS and celiac artery compression, opioid use should be limited or avoided in this patient population.
Strengths of this study include diagnostic imaging with MRA to confirm MALS. During inpatient admission, multiple acupuncture sessions were completed with robust outpatient follow-up at discharge which may point to the efficacy in this patient case.
Limitations include the subjective nature of acupuncture points chosen during each session, although certain points were repeated at each session based on prior literature. Further, although the patient had stopped part of her opioid analgesics, she did rely on a modified analgesic regimen even after surgical intervention. Although the MRA imaging was significant for MALS in the present case, prior studies have demonstrated celiac artery narrowing and/or stenosis in 3.42–24% of asymptomatic individuals (5,18,19). This patient had multiple other comorbidities including IBS and RA which could contribute to her clinical picture, thus multiple etiologies to abdominal pain should be evaluated.
Lastly, we would like to include the patient perspective. She felt that the acupuncture helped to keep her calm in the context of the pain. It also helped to provide adjunct pain reduction and allow her to better cope with her pain.
Acupuncture offers a unique adjunctive treatment to patients before surgical intervention or during the post-operative recovery phase. Although future high-quality research is needed, it is important that clinicians are aware of the available integrative medicine treatment modalities for patients with chronic abdominal pain linked to MALS.
Acknowledgments
Funding: This work was supported by the University of California Los Angeles (UCLA) Center for East-West Medicine.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Longhua Chinese Medicine for the series “UCLA Case Studies”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-24/rc
Peer Review File: Available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-24/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-24/coif). The series “UCLA Case Studies” was commissioned by the editorial office without any funding or sponsorship. GC served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dyches RP, Eaton KJ, Smith HF. The Roles of Celiac Trunk Angle and Vertebral Origin in Median Arcuate Ligament Syndrome. Diagnostics (Basel) 2020;10:76. [Crossref] [PubMed]
- Goodall R, Langridge B, Onida S, et al. Median arcuate ligament syndrome. J Vasc Surg 2020;71:2170-6. [Crossref] [PubMed]
- Kim EN, Lamb K, Relles D, et al. Median Arcuate Ligament Syndrome-Review of This Rare Disease. JAMA Surg 2016;151:471-7. [Crossref] [PubMed]
- Gümüş H, Gümüş M, Tekbaş G, et al. Clinical and multidetector computed tomography findings of patients with median arcuate ligament syndrome. Clin Imaging 2012;36:522-5. [Crossref] [PubMed]
- Petnys A, Puech-Leão P, Zerati AE, et al. Prevalence of signs of celiac axis compression by the median arcuate ligament on computed tomography angiography in asymptomatic patients. J Vasc Surg 2018;68:1782-7. [Crossref] [PubMed]
- Katz-Summercorn A, Bridger J. A cadaveric study of the anatomical variation of the origins of the celiac trunk and the superior mesenteric artery: a role in median arcuate ligament syndrome?. Clin Anat 2013;26:971-4. [Crossref] [PubMed]
- Arazińska A, Polguj M, Wojciechowski A, et al. Median arcuate ligament syndrome: Predictor of ischemic complications? Clin Anat 2016;29:1025-30. [Crossref] [PubMed]
- Weber JM, Boules M, Fong K, et al. Median Arcuate Ligament Syndrome Is Not a Vascular Disease. Ann Vasc Surg 2016;30:22-7. [Crossref] [PubMed]
- Gruber H, Loizides A, Peer S, et al. Ultrasound of the median arcuate ligament syndrome: a new approach to diagnosis. Med Ultrason 2012;14:5-9. [PubMed]
- Berger AA, Liu Y, Jin K, et al. Efficacy of Acupuncture in the Treatment of Chronic Abdominal Pain. Anesth Pain Med 2021;11:e113027. [PubMed]
- Xuefen W, Ping L, Li L, et al. A Clinical Randomized Controlled Trial of Acupuncture Treatment of Gastroparesis Using Different Acupoints. Pain Res Manag 2020;2020:8751958. [Crossref] [PubMed]
- Patel M, Urits I, Kaye AD, et al. The role of acupuncture in the treatment of chronic pain. Best Pract Res Clin Anaesthesiol 2020;34:603-16. [Crossref] [PubMed]
- Kim KH, Lee MS, Choi TY, et al. Acupuncture for symptomatic gastroparesis. Cochrane Database Syst Rev 2018;12:CD009676. [PubMed]
- Li H, He T, Xu Q, et al. Acupuncture and regulation of gastrointestinal function. World J Gastroenterol 2015;21:8304-13. [Crossref] [PubMed]
- Song G, Fiocchi C, Achkar JP. Acupuncture in Inflammatory Bowel Disease. Inflamm Bowel Dis 2019;25:1129-39. [Crossref] [PubMed]
- Theysohn N, Choi KE, Gizewski ER, et al. Acupuncture-related modulation of pain-associated brain networks during electrical pain stimulation: a functional magnetic resonance imaging study. J Altern Complement Med 2014;20:893-900. [Crossref] [PubMed]
- Liu HR, Wang XM, Zhou EH, et al. Acupuncture at both ST25 and ST37 improves the pain threshold of chronic visceral hypersensitivity rats. Neurochem Res 2009;34:1914-8. [Crossref] [PubMed]
- Park CM, Chung JW, Kim HB, et al. Celiac axis stenosis: incidence and etiologies in asymptomatic individuals. Korean J Radiol 2001;2:8-13. [Crossref] [PubMed]
- Levin DC, Baltaxe HA. High incidence of celiac axis narrowing in asymptomatic individuals. Am J Roentgenol Radium Ther Nucl Med 1972;116:426-9. [Crossref] [PubMed]
Cite this article as: Pintas SK, Shubov A, Chu G. Integrative medicine management of median arcuate ligament syndrome: a case report. Longhua Chin Med 2022;5:36.

