
The Glucose Ketone Index predicts overall survival and metastasis of mouse tumor cells to visceral organs and brain
Introduction
Metastasis involves dissemination of tumor cells from the primary tumor to distant tissues (1,2). Metastasis is the primary cause of death among cancer patients, especially after the tumor cells metastasize to the brain (3,4). The VM-M3 is a natural mouse model of human metastatic cancer. We previously showed that the VM-M3/met tumor cells grown in their syngeneic VM/Dk mouse host strain replicate all of the characteristics of the human metastatic cascade to include local invasion, intravasation, immune system survival, immunosuppression, extravasation, secondary tumor formation, and cachexia (2,5-8). Moreover, the VM-M3met cells express multiple biomarkers of macrophages, which are considered the cell of origin for most metastatic cancers regardless of the involved tissue (2,9). Hence, the VM-M3/met is an excellent model for testing therapies against cancer metastasis.
A defining feature of most cancer cells is their dependence on glucose and glutamine fermentation for adenosine triphosphate (ATP) production (10,11). No cell can survive without ATP including cancer cells. Glucose carbons are used for the synthesis of growth metabolites, while glutamine nitrogen and carbons are used for the synthesis of nitrogen-containing metabolites and ATP (10). Defects in the number, structure, and function of tumor cell mitochondria produce respiratory insufficiency thus causing a shift in ATP synthesis from respiration to fermentation (10,12). Like most cancers, the VM-M3/met cells also suffer from respiratory insufficiency due to abnormalities in the content and composition of cardiolipin, a cristae-enriched phospholipid that regulates the electron transport chain (10,13). These cardiolipin abnormalities are linked to reduced activity of electron transport chain activities and a dependency on fermentation metabolism. Consequently, therapeutic strategies that target fermentation should be effective in reducing metastasis (11,12).
Ketogenic metabolic therapy (KMT) reduces glucose-dependent lactic acid fermentation (Warburg effect) in cancer cells while elevating the non-fermentable ketone bodies, D-β-hydroxybutyrate (β-OHB) and acetoacetate. Ketone bodies can replace glucose in cells with normal mitochondria and can also be toxic to tumor cells (12). Moreover, ketone body metabolism enhances the ∆G’ATP hydrolysis in normal cells from −56 to −59 kJ/mole, thus providing normal cells with an energetic advantage over tumor cells (14-16).
We described the Glucose Ketone Index (GKI) as a biomarker for cancer management by tracking glucose and ketone levels in the blood (12,17). In this study, data are presented showing that high GKI values are linked to rapid VM-M3/met/Fluc tumor growth, extensive organ metastasis, and reduced overall survival, whereas low GKI values are linked to slower tumor growth, reduced organ metastasis, especially to brain, and extended survival. Our objective was to determine if low GKI values could be linked to reduced systemic metastatic cancer in the VM-M3/met syngeneic mouse model cancer. We present the following article in accordance with the ARRIVE reporting checklist (available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-43/rc)
Methods
Mice
Mice of the VM/Dk (VM) inbred strain mice were obtained as gifts from H. Fraser (University of Edinburgh, Scotland) and from G. Carlson (McLaughlin Research Institute, Great Falls, Montana). Housing and breeding of the mice used for this study were done in the Boston College Animal Care Facility, utilizing husbandry conditions described elsewhere (6). Experiments were performed under a project license (No. A3905-01) granted by the Institutional Animal Care Committee of Boston College, in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Origin of VM-M3met tumor
The VM-M3 tumor arose spontaneously in the cerebrum of adult VM/Dk mouse, as we described previously (6). The tumor was classified as a glioblastoma (GBM) based on histological appearance and invasive growth behavior in brain (18). The VM-M3 tumor cells also display metastasis to multiple organ systems when given access to extra neural sites (5). The extraneural metastasis seen for the VM-M3 tumor cells is similar to what has been documented for human GBM cells that gain access to extra-neural sites (19-23). The VM-M3 cell line was transduced with a lentivirus vector containing the firefly luciferase gene under control of the cytomegalovirus promoter (VM-M3/Fluc), as we previously described (6). The VM-M3 cells that metastasize from subcutaneous sites to multiple organ systems are labeled as VM-M3/met, and are labeled as “VM-M3/met/Fluc” when expressing bioluminescence. VM-M3/met/Fluc cells express all of the characteristics of the metastatic cascade that has been described for human metastatic cancer (1,2,7).
Subcutaneous implants
VM/Dk mice were anaesthetized with isoflurane to reduce any pain or distress (obtained from Halocarbon, River Edge, NJ, USA). Small VM-M3met/Fluc tumor fragments (about 1 square mm) were implanted subcutaneously into the right flank in 0.2 mL PBS using a 1.0 cc tuberculin syringe attached to an 18-gauge needle, as we described previously (6). Mice were returned to their cages after recovering from the surgical procedure.
Transduction of cell lines and bioluminescent imaging
The VM-M3/met cell line was transduced with a lentivirus vector containing the firefly luciferase gene under the control of the cytomegalovirus promoter (VM-M3/met/Fluc) as we previously described (5,6). Briefly, for in vivo imaging, mice received an i.p. injection of d-luciferin (50 mg/kg) in phosphate buffered saline (PBS) and isoflurane (5% in oxygen). Imaging times ranged from 1 to 5 min, depending on the time point. For ex-vivo imaging, organs were removed and imaged in 0.3 mg d-luciferin in PBS. The IVIS Lumina cooled CCD camera system was used for light acquisition (xenogen imaging). Data acquisition and analysis was performed with Living Image software (Caliper LS).
Diets
The VM/Dk mice received a nutritionally complete standard high carbohydrate PROLAB mouse chow diet (SD) (Agway Inc., NY) that contained carbohydrate, fat, protein, and fiber comprised 62 g, 6 g, 27 g of 100 g of the total diet, respectively. KetoGen ketogenic diet (KG) and vitamin and mineral mix (NanoVM) was a gift of Nancy Moore (Medica Nutrition, Canada, 877-667-6522). The KG contained 2.54 g, 74 g, and 15.17 g of carbohydrate, fat, and protein for 100 g of the total diet, respectively (information provided with personal communication). NanoVM vitamin mix (9.0 g in H2O) was added to 91 g of KetoGen powder to make the complete formula in the form of a paste. No signs of vitamin or mineral deficiency were observed in the R-fed mice according to standard criteria for mice (24). These findings are consistent with the well-recognized health benefits of mild to moderate caloric restriction in rodents (25), and support our previous findings that both the ketogenic diet and a moderate CR are well tolerated by mice (26-28).
Dietary feeding regimens, body weight, and food intake measurements
The dietary feeding regimen that we use for in vivo analysis of tumor growth was designed to provide the highest degree of precision for accurate data interpretation. Approximately 1–2 days before the tumor implantation, adult female and male VM/Dk inbred mice (60–120 days old) were separated and placed in filter-toped plastic cages with Sani-Chip bedding (P.J. Murphy Products Corp., Montville, NJ). Food intake and body weight measurements were performed and recorded daily. Tumor fragment implantation took place on day zero, as we previously described (6). After confirming the presence of a tumor by xenogen imaging, mice were separated into body-weight matched groups, 2–3 days after tumor implantation. All mice were fasted for 18 hr. before diet initiation. Mice on calorie restriction (SD-R or KD-R), received 40–60% of their food intake at around 10:00 hr. In order to reach 18–20% body weight reduction compared to their initial weight, for the total duration of the study. Unrestricted (UR) mice received food ad libitum of their corresponding diet. Daily body weight measurements for all mice were performed prior to food administration.
Measurement of plasma glucose, β-hydroxybutyrate and calculation of GKI
Blood collection from mice was performed prior to sacrifice and tumor resection on the last day of the metastasis study, and on day 14 for the survival study. In order to stabilize blood glucose levels, the mice were fasted for 3 hr. before blood collection, as we previously described (29). Mice were anesthetized with isoflurane (obtained from Halocarbon, NJ) and blood was collected by submandibular bleeding into heparinized tubes. Plasma was collected from whole blood that was centrifuged at 1,500 ×g for 10 minutes, and was stored at 80 ℃ until assayed. The StanBio® Enzymatic Glucose Assay kit (1075-102), and a modification of the Krebs et al., enzymatic procedure was used to spectrophotometrically measure plasma glucose and β-hydroxybutyrate concentrations, respectively (30). For blood ketone body analysis, only β-hydroxybutyrate levels were measured since it is the major blood ketone body in plasma (30,31). The GKI was calculated by dividing the glucose value (mM) by the β-hydroxybutyrate level (mM), as we described previously (17).
Experimental design
VM/Dk mice were implanted subcutaneously with the VM-M3/met/Fluc tumor fragments on Day 0. Mice were fasted for 18 hours on Day 3, and diet was initiated at Day 4 (Figure 1). The tumor growth and metastasis experiment was terminated when mice in the control (SD-UR) group showed signs of morbidity, i.e., lethargy and sudden body weight loss. Diet restricted mice received 40–60% less food compared to unrestricted groups, and reached about 82% of their initial body weights 2–3 days after diet initiation (about 18% body weight reduction). Blood was collected on termination day for analysis of tumor growth and metastasis, whereas blood was collected on day 14 for the survival study.

Statistical analysis
The non-parametric Mann-Whitney U test was used for calculating significance of organ metastasis with photons/sec values, and glucose, ketone and GKI. The survival studies were plotted on a Kaplan-Meir curve using Graph Pad Prizm software and significance was determined using the log-rank test. Pearson correlation and linear regression analysis was done using IBM SPSS 2.1 software. Linear regression was used to determine the relationships among GKI, tumor growth, and metastasis, and overall mouse survival (32).
Results
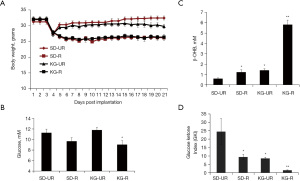
Influence of diet on body weight and circulating levels of glucose, β-OHB, and GKI in tumor-bearing VM/Dk mice
Tumor-bearing VM/Dk mice in the two unrestricted food groups (SD-UR and KG-UR) returned to their initial body weight 2–3 days after diet initiation (Figure 2A). Body weights and blood glucose levels were similar and significantly lower in the SD-R and KG-R groups than in the SD-UR and KG-UR groups, respectively (Figure 2A,2B). Circulating β-OHB levels were significantly higher in the SD-R and KG-R groups than in the SD-UR group (Figure 2C). The β-OHB levels were also significantly higher in KG-R group than in the SD-R and KG-UR groups. The GKI was calculated, as described in methods. The GKI values were significantly lower in the SD-R and the KG-R groups than in the SD-UR group. The GKI values in the KG-R group were significantly lower than those of all other groups (Figure 2D). The findings indicate that food restriction lowers blood glucose levels to a similar degree in the SD-R and the KG-R groups, but food restriction increases blood β-OHB levels to a much greater degree in the KG-R group than in the SD-R group. These findings explain how GKI values are lowest in the KG-R group.
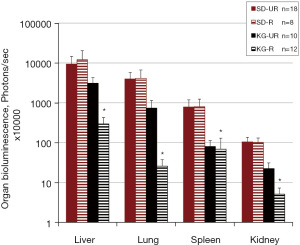
Influence of diet on subcutaneous growth and distant organ metastasis of VM-M3/met/Fluc tumor cells
Primary tumor size was significantly smaller in the SD-R, KG-UR and KG-R groups than in the control (SD-UR) group (Figure 3A,3B). Also, primary tumor weight was significantly lower in the KG-R group than in the SD-R and KG-UR groups. Metastasis of the VM-M3/met/Fluc tumor cells to liver, lung, spleen and kidney was significantly less in the KG-R group than in SD-UR and SD-R groups (Figure 4). SD-R or KG-UR groups did not reduce the metastasis to organs compared to control SD-UR mice (Figure 4).

Influence of diet on survival of VM-M3/met tumor bearing mice
The time to morbidity (lethargy and sudden body weight loss) after tumor implantation occurred between 18–22 days post implantation in the SD-UR group (Figure 5). Mice in the KG-R group survived longer than mice in the SD-R and KG-UR groups. No mouse survived beyond 35 days after implantation.

GKI predicts tumor growth and survival
To determine whether the GKI values were predictive of tumor growth and overall mouse survival, we analyzed the data using simple linear regression, as previously described (26). The simple linear regression analysis showed that the GKI was predictive of tumor growth and survival (Figure 6A,6B). In each regression analysis, the GKI was designated as the independent (causative) variable and tumor growth (weight) and mouse survival (days) were designated as the dependent variables. Low GKI values obtained with low glucose and high ketone values delivered the best therapeutic outcome. The GKI had a significant positive association with tumor growth; meaning that lower GKI values predicted reduced tumor growth (weight) while high GKI values predicted faster tumor growth (Figure 6A). On the other hand, the GKI had a significant negative association with overall survival (days); meaning that low GKI values were associated with longer survival while high GKI values were associate with shorter survival (Figure 6B). A correlation analysis also showed that the degree of metastasis to liver, lung, spleen, and kidney was less in mice with low GKI values than in mice with high GKI values (Table 1).
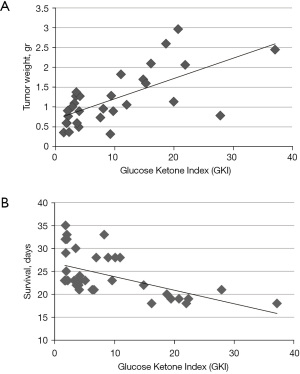
Table 1
| GKI | Liver | Lung | Spleen | Kidney |
|---|---|---|---|---|
| Pearson correlation | 0.58 | 0.72 | 0.721 | 0.79 |
| Sig (2-tailed) | 0.03* | 0.004* | 0.004* | 0.001* |
*, P<0.05. GKI, Glucose Ketone Index.
Influence of diet on metastasis of VM-M3/met/Fluc tumor cells to the brain
Brain bioluminescence was significantly lower in mice fed the KG-R than in the mice fed SD-UR (Figure 7). The other groups were not included in this separate study that involved 6 mice/group. The blood glucose (mM), β-OHB (mM), and GKI values (± SEM) for the mice in the SD-UR group were 11.1±0.4, 0.21±0.08, and 52.8±4.51, respectively. The blood glucose (mM), β-OHB (mM), and GKI values (± SEM) for the mice in the KG-R group were 8.75±0.4, 3.4±0.2, and 2.6±1.9, respectively. These data show that metastasis of the VM-M3/met/Fluc tumor cells to the brain and GKI levels were significantly lower in the mice fed the KG-R than in mice fed the SD-UR. Study data are available upon request.

Discussion
Cancer kills more people than heart disease in every age group except in the 65+ years group. Despite decades of extensive research, only a 2.4-month increase in overall survival has been achieved using approved drugs for advanced cancers (33). Most conventional therapies, such as radiation and chemotherapy, are unacceptably toxic and do not target tumor cells specifically. Immunotherapies can cause hyperaggressive disease accelerating the death of some cancer patients (34,35). Since most cancer cells express increased glucose and glutamine fermentation due to defective OxPhos, success in managing cancer can come from therapeutic strategies that target the availability of glucose and glutamine (10,12,36).
In this study, we analyzed the influence of diet on the growth and metastasis of VM-M3/met/Fluc tumor cells grown in their natural syngeneic VM/Dk host strain. The diets included a standard high-carbohydrate mouse chow diet (SD) and a low-carbohydrate, high-fat ketogenic diet (KG). The diets were fed to the mice in unrestricted amounts (SD-UR, KG-UR) or in restricted amounts (SD-R, KG-R) to achieve similar body weights within groups. Body weight matching within dietary groups is essential for accurate data interpretation in dietary studies (26,29,37). The most rapid tumor growth, the shortest overall survival, and the greatest organ metastasis was seen in the mice fed the SD-UR. On the other hand, the slowest tumor growth, longest overall survival, and the least organ metastasis was seen in the mice fed the KG-R. The therapeutic benefit of the KG-R was linked directly to the GKI, which was lower in this group than in the other diet groups. Simple linear regression analysis also showed that the GKI could predict tumor growth and mouse survival. Low GKI values were also linked to reduced VM-M3/met/Fluc tumor cell metastasis to the brain. This is important, as secondary metastasis to the brain is linked to the poorest overall survival in cancer patients (3,4).
Our findings of a therapeutic effect of reduced glucose and elevated ketones on systemic metastasis in this model are also in agreement with our previous findings in other preclinical models of brain cancer (38-40). Our findings are also consistent with the recent findings of the Hagihara et al. group showing that low GKI values are linked to better survival and quality of life for patients with various cancers than are higher GKI values (41). We found that reduced glucose and elevated ketones not only kills tumor cells, but also reduces tumor angiogenesis and inflammation in the tumor microenvironment (39,40,42,43). We predict that similar findings will be seen in the tumors of cancer patients that can maintain low GKI values (44).
The GKI was recently associated with improved survival in patients with glioblastoma, but further studies with larger patient numbers would be required to determine if the GKI could be predictive of improved survival in GBM patients (14,45). Due to differences in basal metabolic rate in mice and humans, a 40% calorie restriction in mice is comparable to water-only fasting in humans (46). We suggested that GKI values in the range of 2.0 or below would be best for managing human cancers (14,17). These values can be achieved with either calorie restricted KG or with water-only fasting for at least 5–7 days. It is important to recognize that blood glucose can be reduced to very low levels (0.5 mM or 9 mg/dL) as long as ketone bodies are elevated (47). Ketone body elevation becomes more therapeutic when total calories become restricted. Therapeutic outcome is best under low glucose levels and elevated ketone levels, which also reduces systemic inflammation (42). The GKI captures the therapeutic value of both reduced glucose and elevated ketone bodies. Low GKI values might be difficult to achieve for many cancer patients, however, especially for those treated with glucose-elevating steroids or with toxic therapies that increase systemic inflammation. KMT used with lower doses of chemotherapy has demonstrated improved survival over traditional standard of care for breast cancer and lung cancer (48-51). Further clinical studies will be needed to determine if low GKI values can be linked to improved progression free and overall survival for patients with most types of cancers.
The mechanism by which low GKI values can manage cancer has been described (12). A limitation of the present study involves a single syngeneic model of systemic metastatic cancer. Nevertheless, it is well known that reduced glucose levels will target aerobic glycolysis (Warburg effect), which drives the growth of most malignant cancers (10,52). Glucose carbons are essential for metabolite synthesis in cancer cells. As ketone bodies and fatty acids are non-fermentable, they cannot serve as alternative fuels to glucose and glutamine for tumor growth (10). Importantly, ketone body metabolism enhances respiratory energy efficiency in cells with normal mitochondria (16). Calorie restriction will reduce angiogenesis and inflammation in the tumor microenvironment, as referenced above. Impaired mitochondrial respiration prevents efficient metabolism of ketone bodies in tumor cells. Elevated ketone bodies will further reduce glucose levels and can indirectly target the glutaminolysis pathway that is necessary of ATP synthesis (11,14).
Conclusions
As there is no known cancer drug that can target tumor cells, while also reducing systemic inflammation and enhancing the energy efficiency of normal cells, the GKI becomes a compelling biomarker for managing a broad range of metastatic and invasive cancers.
Acknowledgments
We thank Derek Lee for assistance with the statistical tests used for data analysis.
Funding: We thank the Foundation for Metabolic Cancer Therapies, CrossFit Inc., The Nelson and Claudia Peltz Family Foundation, Lewis Topper, The John and Kathy Garcia Foundation, Edward Miller, Dr. Joseph Maroon, the Kenneth Rainin Foundation, Children with Cancer UK, the Robert L. Corkin Charitable Foundation, and the Boston College Research Expense Fund for their support. None of the funders had a role in the design, analysis, and reporting of the study.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-43/rc
Data Sharing Statement: Available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-43/dss
Peer Review File: Available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-43/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://lcm.amegroups.com/article/view/10.21037/lcm-21-43/coif). The authors report that they received funding from Foundation for Metabolic Cancer Therapies for their major research support including animal expenses, salaries, supplies etc.; a personal donation from Edward Miller goes to Foundation for Metabolic Cancer Therapies. Cross Fit had a major funding for role of exercise in prevention and progression of cancer in their mice models. Lewis Topper, cancer UK grant and others directly send funding to Boston College for their research. They do not have any conflicts of interest like royalties or consulting fees from any of the funders of this research mentioned above. They have assigned money from Children with Cancer UK, for meeting attend and manuscript processing fees. TNS and PM has a planned patent on diet/drug therapy for cancer.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer 2003;3:453-8. [Crossref] [PubMed]
- Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog 2013;18:43-73. [Crossref] [PubMed]
- Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers 2019;5:5. [Crossref] [PubMed]
- Langley RR, Fidler IJ. The biology of brain metastasis. Clin Chem 2013;59:180-9. [Crossref] [PubMed]
- Huysentruyt LC, Shelton LM, Seyfried TN. Influence of methotrexate and cisplatin on tumor progression and survival in the VM mouse model of systemic metastatic cancer. Int J Cancer 2010;126:65-72. [Crossref] [PubMed]
- Huysentruyt LC, Mukherjee P, Banerjee D, et al. Metastatic cancer cells with macrophage properties: evidence from a new murine tumor model. Int J Cancer 2008;123:73-84. [Crossref] [PubMed]
- Taus LJ, Flores RE, Seyfried TN. Quantification of metastatic load in a syngeneic murine model of metastasis. Cancer Lett 2017;405:56-62. [Crossref] [PubMed]
- Koutnik AP, Poff AM, Ward NP, et al. Ketone Bodies Attenuate Wasting in Models of Atrophy. J Cachexia Sarcopenia Muscle 2020;11:973-96. [Crossref] [PubMed]
- Huysentruyt LC, Seyfried TN. Perspectives on the mesenchymal origin of metastatic cancer. Cancer Metastasis Rev 2010;29:695-707. [Crossref] [PubMed]
- Seyfried TN, Arismendi-Morillo G, Mukherjee P, et al. On the Origin of ATP Synthesis in Cancer. iScience 2020;23:101761. [Crossref] [PubMed]
- Seyfried TN, Chinopoulos C. Can the Mitochondrial Metabolic Theory Explain Better the Origin and Management of Cancer than Can the Somatic Mutation Theory? Metabolites 2021;11:572. [Crossref] [PubMed]
- Seyfried TN, Yu G, Maroon JC, et al. Press-pulse: a novel therapeutic strategy for the metabolic management of cancer. Nutr Metab (Lond) 2017;14:19. [Crossref] [PubMed]
- Kiebish MA, Han X, Cheng H, et al. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J Lipid Res 2008;49:2545-56. [Crossref] [PubMed]
- Seyfried TN, Shivane AG, Kalamian M, et al. Ketogenic Metabolic Therapy, Without Chemo or Radiation, for the Long-Term Management of IDH1-Mutant Glioblastoma: An 80-Month Follow-Up Case Report. Front Nutr 2021;8:682243. [Crossref] [PubMed]
- Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids 2004;70:309-19. [Crossref] [PubMed]
- Veech RL, Todd King M, Pawlosky R, et al. The "great" controlling nucleotide coenzymes. IUBMB Life 2019;71:565-79. [Crossref] [PubMed]
- Meidenbauer JJ, Mukherjee P, Seyfried TN. The glucose ketone index calculator: a simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr Metab (Lond) 2015;12:12. [Crossref] [PubMed]
- Shelton LM, Mukherjee P, Huysentruyt LC, et al. A novel pre-clinical in vivo mouse model for malignant brain tumor growth and invasion. J Neurooncol 2010;99:165-76. [Crossref] [PubMed]
- Hamilton JD, Rapp M, Schneiderhan T, et al. Glioblastoma multiforme metastasis outside the CNS: three case reports and possible mechanisms of escape. J Clin Oncol 2014;32:e80-4. [Crossref] [PubMed]
- Hoffman HJ, Duffner PK. Extraneural metastases of central nervous system tumors. Cancer 1985;56:1778-82. [Crossref] [PubMed]
- Xu M, Wang Y, Xu J, et al. Extensive Therapies for Extraneural Metastases from Glioblastoma, as Confirmed with the OncoScan Assay. World Neurosurg 2016;90:698.e7-698.e11. [Crossref] [PubMed]
- Yasuhara T, Tamiya T, Meguro T, et al. Glioblastoma with metastasis to the spleen--case report. Neurol Med Chir (Tokyo) 2003;43:452-6. [Crossref] [PubMed]
- Kalokhe G, Grimm SA, Chandler JP, et al. Metastatic glioblastoma: case presentations and a review of the literature. J Neurooncol 2012;107:21-7. [Crossref] [PubMed]
- Hoag WG, Dickie MM. Nutrition. In: Green EL. editor. Biology of the Laboratory Mouse. 2nd ed. New York: Dover, 1968.
- Keenan KP, Ballam GC, Soper KA, et al. Diet, caloric restriction, and the rodent bioassay. Toxicol Sci 1999;52:24-34. [Crossref] [PubMed]
- Seyfried TN, Sanderson TM, El-Abbadi MM, et al. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer 2003;89:1375-82. [Crossref] [PubMed]
- Todorova MT, Tandon P, Madore RA, et al. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia 2000;41:933-40. [Crossref] [PubMed]
- Greene AE, Todorova MT, McGowan R, et al. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia 2001;42:1371-8. [Crossref] [PubMed]
- Meidenbauer JJ, Ta N, Seyfried TN. Influence of a ketogenic diet, fish-oil, and calorie restriction on plasma metabolites and lipids in C57BL/6J mice. Nutr Metab (Lond) 2014;11:23. [Crossref] [PubMed]
- Krebs HA, Williamson DH, Bates MW, et al. The role of ketone bodies in caloric homeostasis. Adv Enzyme Reg 1971;9:387-409. [Crossref]
- VanItallie TB, Nufert TH. Ketones: metabolism's ugly duckling. Nutr Rev 2003;61:327-41. [Crossref] [PubMed]
- Lang TA, Secic M. How to Report Statistics in Medicine. Philadelphia: Amer. College Physicians, 1997.
- Ladanie A, Schmitt AM, Speich B, et al. Clinical Trial Evidence Supporting US Food and Drug Administration Approval of Novel Cancer Therapies Between 2000 and 2016. JAMA Netw Open 2020;3:e2024406. [Crossref] [PubMed]
- Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol 2018;4:1543-52. [Crossref] [PubMed]
- Adashek JJ, Kato S, Ferrara R, et al. Hyperprogression and Immune Checkpoint Inhibitors: Hype or Progress? Oncologist. 2020;25:94-8. [Crossref] [PubMed]
- Seyfried TN, Mukherjee P, Iyikesici MS, et al. Consideration of Ketogenic Metabolic Therapy as a Complementary or Alternative Approach for Managing Breast Cancer. Front Nutr 2020;7:21. [Crossref] [PubMed]
- Mattson MP, Allison DB, Fontana L, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A 2014;111:16647-53. [Crossref] [PubMed]
- Zhou W, Mukherjee P, Kiebish MA, et al. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 2007;4:5. [Crossref] [PubMed]
- Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clin Cancer Res 2004;10:5622-9. [Crossref] [PubMed]
- Mukherjee P, El-Abbadi MM, Kasperzyk JL, et al. Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br J Cancer 2002;86:1615-21. [Crossref] [PubMed]
- Hagihara K, Kajimoto K, Osaga S, et al. Promising Effect of a New Ketogenic Diet Regimen in Patients with Advanced Cancer. Nutrients 2020;12:1473. [Crossref] [PubMed]
- Mulrooney TJ, Marsh J, Urits I, et al. Influence of caloric restriction on constitutive expression of NF-κB in an experimental mouse astrocytoma. PLoS One 2011;6:e18085. [Crossref] [PubMed]
- Urits I, Mukherjee P, Meidenbauer J, et al. Dietary restriction promotes vessel maturation in a mouse astrocytoma. J Oncol 2012;2012:264039. [Crossref] [PubMed]
- Weber DD, Aminzadeh-Gohari S, Tulipan J, et al. Ketogenic diet in the treatment of cancer - Where do we stand? Mol Metab 2020;33:102-21. [Crossref] [PubMed]
- Elsakka AMA, Bary MA, Abdelzaher E, et al. Management of Glioblastoma Multiforme in a Patient Treated With Ketogenic Metabolic Therapy and Modified Standard of Care: A 24-Month Follow-Up. Front Nutr 2018;5:20. [Crossref] [PubMed]
- Mahoney LB, Denny CA, Seyfried TN. Caloric restriction in C57BL/6J mice mimics therapeutic fasting in humans. Lipids Health Dis 2006;5:13. [Crossref] [PubMed]
- Drenick EJ, Alvarez LC, Tamasi GC, et al. Resistance to symptomatic insulin reactions after fasting. J Clin Invest 1972;51:2757-62. [Crossref] [PubMed]
- Iyikesici MS. Feasibility study of metabolically supported chemotherapy with weekly carboplatin/paclitaxel combined with ketogenic diet, hyperthermia and hyperbaric oxygen therapy in metastatic non-small cell lung cancer. Int J Hyperthermia 2019;36:446-55. [Crossref] [PubMed]
- İyikesici MS, Slocum AK, Slocum A, et al. Efficacy of Metabolically Supported Chemotherapy Combined with Ketogenic Diet, Hyperthermia, and Hyperbaric Oxygen Therapy for Stage IV Triple-Negative Breast Cancer. Cureus 2017;9:e1445. [Crossref] [PubMed]
- İyikesici MS, Slocum AK, Winters N, et al. Metabolically Supported Chemotherapy for Managing End-Stage Breast Cancer: A Complete and Durable Response. Cureus 2021;13:e14686. [Crossref] [PubMed]
- Khodabakhshi A, Akbari ME, Mirzaei HR, et al. Effects of Ketogenic metabolic therapy on patients with breast cancer: A randomized controlled clinical trial. Clin Nutr 2021;40:751-8. [Crossref] [PubMed]
- Yu M, Chen S, Hong W, et al. Prognostic role of glycolysis for cancer outcome: evidence from 86 studies. J Cancer Res Clin Oncol 2019;145:967-99. [Crossref] [PubMed]
Cite this article as: Akgoc Z, Mukherjee P, Seyfried TN. The Glucose Ketone Index predicts overall survival and metastasis of mouse tumor cells to visceral organs and brain. Longhua Chin Med 2022;5:12.

