
Saffron (Crocus sativus L.) as a valuable spice and food product: a narrative review
Introduction
Crocus sativus L. belongs to the Iridaceae family and is a perennial crop of monocotyledonous plants that form bulbs. It is planted around the end of summer in well-drained soil. The height of the flower stalk is approximately 100–150 mm, and it has six pale purple tepals, which grow to a size of approximately 30 mm in fall. The plant has three yellow stamens, a stigma, and a pistil of approximately 20, 20–40, and 60–100 mm in length, respectively. The stigma extending like a trumpet is divided into three parts and has a bright orange-red color. The dried stigma of this plant is commonly known as saffron (Figure 1), which is derived from the Arabic word “Zafaran”, meaning yellow. However, a saffron-like horticultural plant belonging to the Colchicaceae family, Colchicum autumnale, contains colchicine, a tropolone alkaloid (1). It is used as a pharmaceutical resource and is not edible. Several cases of poisoning and death due to accidental ingestion of parts of this plant have been reported, because its bulbs and leaves resemble those of onions and garlic plants (2-6).
Saffron is known as one of the oldest cultivated plants, although its wild species is unknown. Historically, saffron has been traced back to cave arts in Mesopotamia, dating back at least 5,000 years (7). It was cultivated from 2500 to 1500 B.C.E. (8). Although the origin of its cultivation is unclear, it is found to have been cultivated in Crete and Thera during the Minoan period (7,9). Saffron is believed to have originated from ancient Greece, Asia Minor, and Persia. The saffron spice and its cultivation later spread to India and China through the Silk Road across civilizations, cultures, continents, and countries. Saffron is currently cultivated in many countries including Iran, Spain, India, Italy, Afghanistan, Azerbaijan, China, and Uzbekistan. The major saffron-producing countries are Iran and Spain, which mainly export to Europe and Asia (10). In Japan, the main area of production has been Taketa City in the Oita Prefecture since approximately 1903, where the cultivated saffron is of good quality because the cultivation areas are indoors and not affected by weather (11).
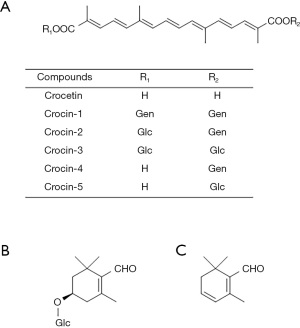
In general, saffron is one of the most valuable spices and is used as a flavoring, coloring, and aroma agent in food and drinks worldwide (12). Saffron is consumed in the food industry and is also used in fragrances, cosmetics, and dyes (7,12,13). Furthermore, saffron has been used in traditional medicine, crude drug, and folk medicine since ancient times because of its therapeutic properties (14). The attractive functions of saffron depend on its main compounds: crocins, picrocrocin, and safranal (15) (Figure 2A-2C).
Saffron is sometimes called “red gold” because it is one of the most expensive cash crops (16). It costs approximately 10 times more than vanilla and 50 times more than cardamom. Cultivation using non-mechanized agricultural systems, harvesting, and processing require complicated skills such as manual removal of the stigma on the day of harvest and approximately 400 h of work (17,18). Moreover, saffron is a very low-yield crop. Approximately 70,000–200,000 flower stigmas are required to produce 1 kg of saffron (7,13,19); one stigma formed by three filaments is obtained from each flower.
Currently, there are many barriers against saffron usage to the widespread use of saffron in the food industry, such as low productivity cultivation methods, production difficulties, low yields, quality control, and food safety concerns. Therefore, it is necessary to provide and organize information on these issues from a broad food perspective. Therefore, this review focuses on saffron in the food industry, its consumption as a spice, its effects on the economy, its ingredients, quality, safety, and its use in cooking. We present the following article in accordance with the Narrative Review reporting checklist (available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-1/rc).
Methods
This narrative review article was constructed from published scholarly papers obtained from various web databases, including PubMed, Google Scholar, ScienceDirect, and Springer, as well as from websites and published books. Our search period for this article was from October 2021 to December 2021. Searches were made using “saffron” or terms related to saffron as the keywords. Non-English and unpublished articles were excluded. This study strategy is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | From October 1, 2021 to December 31, 2021 |
| Databases and other sources searched | PubMed, Google Scholar, ScienceDirect, Springer, websites, and published books |
| Search terms used (including MeSH and free text search terms and filters) | “Saffron”, “Crocus sativus”, “saffron spice”, “Crocins”, “picrocrocin”, “safranal”, “saffron production”, “safety and toxicity of saffron”, “trade of saffron”, “saffron cuisine”, and “traditional food” |
| Timeframe | Publication between 1982 and 2021 |
| Inclusion and exclusion criteria (study type, language restrictions, etc.) | Inclusion criteria: studies about saffron as a multifaceted food perspective, including safety, production, trade, standardization, and utilization (only English-language articles) |
| Exclusion criteria: non-English and unpublished studies | |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Shunsuke Fujii, Yumi Morita, and Tomoe Ohta conducted the selection of references together. A consensus has been obtained from all members of the authors |
| Any additional considerations, if applicable | None |
World trade of saffron
Saffron is cultivated and produced in Asia, the Middle East, and Europe and exported worldwide. Approximately 200–300 tons of saffron are produced globally every year (12,20). Table 2 shows the world trade value of saffron. In 2019, saffron trading rank was 3,550 with a trade value of $229 million (10), accounting for 0.0013% of all global trade. In 2019, Iran and Spain were the top exporters of saffron, claiming more than half of the global trade. Spain and Hong Kong were the top importers. Furthermore, between 2018 and 2019, the scale of saffron exports expanded rapidly in Afghanistan, Uzbekistan, Netherlands, France, and United Kingdom. In 2018, the average tariff for saffron was 14.3%; the country with the highest tariff was Iran (55%) given that it is a major exporter, thus, it has been imposed protective tariffs.
Table 2
| Exporting country/region | Export value, $million | Importing country/region | Import value, $million |
|---|---|---|---|
| Iran | 102 (44.5) | Spain | 34.1 (14.9) |
| Spain | 49.8 (21.8) | Hong Kong | 33.6 (14.7) |
| Afghanistan | 28.9 (12.6) | Saudi Arabia | 19.8 (8.65) |
| China | 5.73 (2.50) | United Arab Emirates | 18.6 (8.12) |
| Hong Kong | 5.48 (2.40) | India | 18.3 (8.01) |
| Uzbekistan | 5.12 (2.24) | United States | 16.3 (7.11) |
| Greece | 4.8 (2.08) | Italy | 10.7 (4.66) |
| France | 4.2 (1.84) | France | 8.5 (3.70) |
| Netherlands | 3.74 (1.63) | Sweden | 7.17 (3.13) |
| Portugal | 3.47 (1.52) | Kuwait | 5.71 (2.50) |
| Poland | 2.32 (1.01) | Germany | 5.55 (2.43) |
| Germany | 1.97 (0.86) | Turkey | 5.41 (2.36) |
| Italy | 0.947 (0.41) | United Kingdom | 4.29 (1.87) |
| Sweden | 0.9 (0.39) | Switzerland | 4.02 (1.76) |
| United Kingdom | 0.852 (0.37) | China | 4.00 (1.75) |
Numerals in parentheses indicate percentages of total export or import value.
However, the production of saffron is declining due to bacterial contamination in one of the major production areas, Kashmir, India (21). Moreover, since after 2020, exporter growth has decreased (−41%) because of the SARS-CoV-2 (COVID-19) pandemic that brought about decreased consumer spending. Thus, the pandemic poses a threat to the world trade of saffron.
Profile of saffron components
The profile of the components, including nutrients and secondary metabolites of saffron, have been investigated in various studies. More than 150 compounds have been isolated from saffron (22,23). Saffron contains nutrients and other constituents, such as water, carbohydrates, fats, proteins, vitamins (riboflavin and thiamine), minerals, ash, dietary fiber, organic acid, anthocyanins, flavonoids, and carotenoids (14,24-26). These components are not involved in the quality considerations of saffron, except for moisture content, ash, and carotenoids such as crocins. Serrano-Díaz et al. reported the nutrient composition of saffron, as shown in Table 3. In their study, the moisture content was changed by the drying method and dehydration temperature (25). In addition, the composition of minerals, including phosphorus and potassium, was shown to be affected by the soil conditions of the growing field. The nutrients identified in the study have various health benefits (25). However, the contribution of the nutrients to human health is low because saffron is consumed as a spice or in food in very small quantities.
Table 3
| Components | Composition (g/100 g dry weight) | Components | Composition (g/100 g dry weight) |
|---|---|---|---|
| Energy (kcal) | 381.2 | Dietary fiber | |
| Moisture | 3.84 | Total | 13.8 |
| Ash | 6.60 | Insoluble | 7.8 |
| Minerals | Soluble | 6.0 | |
| P | 0.327 | Soluble sugars | |
| Mg | 0.135 | Glucose | 7.40 |
| Ca | 0.107 | Fructose | 0.39 |
| Fe | 0.011 | Sucrose | n.d. |
| K | 1.486 | Maltose | n.d. |
| Na | 0.010 | Sugar alcohols | |
| Protein | 13.63 | Inositol | 0.33 |
| Lipid | 8.76 | Sorbitol | 0.20 |
| Available carbohydrate | 62.0 | Mannitol | n.d. |
| Reducing sugar | 16.5 |
This table is a summary and partially extract version of the table of analytical values reported by Serrano-Díaz et al. (25). n.d.; not detected.
Among secondary metabolites of saffron, the well-known components are crocins, picrocrocin, and safranal, which are important as coloring, flavoring, and aroma agents in cooking. Crocetin, the aglycone of crocins, is a major component of the saffron C20 apocarotenoids, and safranal and picrocrocin are secondary metabolites that play an important role in the food industry, cosmetics, and medicine. Crocins, crocetin ester derivatives, are found in saffron and in the fruit of Gardenia jasminoides. These compounds are the main coloring and bioactive constituents of saffron. Five types of crocin derivatives are known as crocin-1, crocin-2, crocin-3, crocin-4, and crocin-5 that are distinguished by the number and position of the sugar moieties bound to the crocetin (27) (Figure 2A). Crocin-1, the main component, comprises two gentiobiose groups bound to crocetin. Crocins are also distinguished into two stereoisomers, where all double bonds in the crocetin chain are trans and the other is formed with one cis double bond on C13 of the chain. Carmona et al. have identified 15 crocins in saffron (28). Although carotenoids are generally classified as lipophilic, crocins are hydrophilic carotenoids. Crocins contain water-soluble gentiobiose molecules. Several highly polar oxygen atoms are present in gentiobiose molecules. Therefore, they are highly water-soluble because the oxygen atoms in gentiobiose form hydrogen bonds. Although picrocrocin, which is reported to contain up to approximately 26% in dry saffron (29), is an odorless and colorless constituent, it has a bitter taste and is important for the taste of saffron. Picrocrocin is a degradation compound of zeaxanthin in saffron and yields 2 mol from 1 mol crocins (30). In an aqueous extract of saffron, the degradation of picrocrocin is more stable than that of crocetin esters (31). During the drying process, picrocrocin releases its glucose molecule, which generates safranal, a major volatile compound of saffron (occupying 65% of the total aroma constituents (15) in more than 60 volatile components of saffron by dehydration. This reaction involves the enzyme glucosidase or thermal degradation (30). Thus, crocins, picrocrocin, and safranal are employed as quality control markers for saffron by the International Organization for Standardization (ISO).
Quality management and storage condition of saffron
Numerous factors affect the quality of saffron, including cultivation conditions, harvesting techniques, post-harvesting processing, and storage conditions. Saffron carotenoids are degraded by the effects of light, heat, and oxygen during storage (19,32-34). For example, Tong et al. reported that saffron lost 52.2% and 54.3% of carotenoids and picrocrocin, respectively, within one year of storage. Moreover, both components decreased to approximately 1.0% after 15 years of storage (35). This section reviews the relationship between moisture and saffron carotenoids in post-harvest processing and storage conditions.
Serrano-Díaz et al. reported that the amount of moisture in the fresh stigmas of saffron is 80.0% (25). After harvesting, saffron is dehydrated to approximately 10% moisture content to maintain its quality. However, moisture content varies depending on the dehydration method and temperature effects (25). Raina et al. investigated the relationship between the dehydration technique, time, and the quality of saffron in detail. Drying temperatures that were below 30 ℃ or excessively high resulted in poor quality saffron due to biodegradation (enzymatic hydration) or decrease of crocins, respectively. Moreover, when saffron was processed in a vacuum oven, crocins were retained, but 4-β-hydroxy safranal, which is an intermediate in the formation of safranal, was obtained as the major component (36). Considering saffron quality (color and flavor), the appropriate drying conditions are in a solar cabinet or oven at 40 ℃ for 6.3 h (19). Moreover, water activity plays a key role in saffron quality because it affects the color and flavor of saffron. It has been reported that water activity (0.4–0.53) produces safranal while suppressing the degradation of saffron carotenoids (37). Based on these studies, the authors suggest that freeze-drying, which is a simple pretreatment, is among the optimal techniques for the dehydration of saffron.
Moisture control is important for the storage of saffron, and long-term storage affects the color and flavor of saffron (35). If the moisture level is maintained at approximately 5%, degradation of saffron carotenoids can be suppressed (36). The quality of saffron is better maintained under dark conditions than under light or refrigeration (0 ℃) (38). In addition, Morimoto et al. recommended the storage of saffron at −20 ℃ to ensure retention of its pharmacological activity (39). Recently, various research fields have developed technologies to maintain saffron quality. For example, coated saffron, which uses a carbohydrate polymer, maltodextrin, in combination with nanocellulose fiber, suppresses hydrolysis by preventing water penetration (40). Moreover, microencapsulation by spray drying using biopolymers such as maltodextrin, Gum Arabic, and gelatin protects the major saffron components: picrocrocin, safranal, and crocins (41). Additionally, tissue culture has been utilized to produce saffron in an attempt to obviate the need for vast amounts of farmland to yield a large harvest of flowers. However, the research and its techniques are still in development (42-45).
Collaborative research on saffron storage methods has been performed within various disciplines using evolving technologies. These techniques are useful in saffron food processing, and their use will be expanded. Furthermore, tissue culture technology may ensure a stable supply of high-quality saffron in the future. However, the techniques have not yet been established or adopted. Furthermore, it is also necessary to overcome crocin and picrocrocin content problems and ensure the safety of saffron produced by tissue culture.
Standardization of saffron
The saffron harvest process is tedious as it is done manually, given that the crocins and picrocrocin content reach a maximum during the full bloom term (39). As saffron is one of the most expensive spices in the international food market depending on its quality and purity, it is prone to fraud (46). Several counterfeits have been reported; for instance, safflower, turmeric, gardenia fruits, and stamens of Crocus sativus were found to be added to saffron (28,47,48). Therefore, various analytical methods have been developed for ingredient analysis and quality control of saffron (49-54).
The standardization of saffron varies with the country of production. Super Negin, Coupé, and Mongra are equivalent to the highest quality products in Iran, Spain, and India, respectively (7,25). The harvested saffron from different countries is graded and accordingly categorized depending on the appearance of the stigma, harvesting, and processing techniques. In particular, the Iranian (Persian) saffron, which accounts for the majority of the global supply, is categorized in order of quality as follows: Super Negin, Sargol, Negin, and Poshal (7). However, combining components other than the stigma, such as yellow threads (styles) or mixed stamens, lowers the quality. Saffron that is categorized as Super Negin has all red components and no yellow components. In some cases, the quality classification of saffron is based on knowledge and experience (organoleptic test). For example, real saffron will not fade when immersed in water. In addition, pure saffron has a clean and sweet odor; however, the taste is not sweet but bitter.
The abovementioned quality control approaches may not determine the quality of saffron in detail or with complete clarity. Therefore, in 1993, the first edition of the standardization system for saffron was established by the ISO. ISO3632 was applied to the international saffron market as a universal standard. The standard was further tightened between 2010–2011. In particular, the classification was performed by measuring the quality control markers using spectrophotometry in ultraviolet (UV) at 257 nm (picrocrocin as flavor), 330 nm (safranal as aroma), and 440 nm (crocins as color), which are categorized into three types in detail, as shown in Table 4 (55). However, these analytical methods have several limitations for the determination of saffron quality. The determination of safranal, the substance responsible for the aroma of saffron, is essential for its quality control because safranal is specific to saffron. Crocins are also present in gardenia fruit. Safranal content has been determined using UV-vis spectrophotometry at a wavelength of 330 nm with a water-soluble extract as the analyte. In the method employed in ISO 3632, some crocetin esters (mainly cis-isomers) are reported to interfere with the analysis of safranal, because they have an absorption wavelength of near 330 nm (28,56,57). However, the degree of interference is not clear. In addition, while crocins and picrocrocin are graded according to their content, the maximum and minimum amount of safranal is the same for all grade categories. Therefore, safranal, which importantly determines the aroma of saffron, is not reflected with high precision in the saffron grade classification, and the ISO 3632 standard cannot be said to accurately reflect the quality of saffron (56). In fact, a quantitative analysis method for safranal contents using a high performance liquid chromatography (HPLC) coupled diode array detector (HPLC-DAD) has been reported for the quality control of saffron (57), furthermore, a comparison of its result with those of the quantitative analysis method used in ISO 3632 reveals that there is no correlation (58). They concluded that the HPLC-DAD method is suitable for the quality assessment of saffron, but there is a limitation: safranal is easily decomposed and the purity of the safranal reagent is low or unclear. In the method using an absolute calibration curve, the reliability of the calibration curve is poor due to the degradation of safranal. Although an analytical method has also been developed for the determination of these quality control markers using liquid chromatography tandem-mass spectrometry, this method has not been widely tested, and thus, no effective method has been established. Therefore, a simple and accurate discrimination method must be developed, which can establish a definitive analytical methodology in the future.
Table 4
| Characteristics | Specification categories | ||
|---|---|---|---|
| I | II | III | |
| Moisture and volatile contents (mass fraction), % max. | |||
| Filaments and cut filaments forms | 12 | 12 | 12 |
| Powder form | 10 | 10 | 10 |
| Total ash (mass), on dry matter, % max. | 8 | 8 | 8 |
| Soluble extract in cold water (mass fraction), on dry matter, % max. | 65 | 65 | 65 |
| Flavor strength (expressed as picrocrocin) | |||
| 257 nm, on dry matter, min. | 70 | 55 | 40 |
| Aroma strength (expressed as safranal) | |||
| 330 nm, on dry matter | |||
| Min. | 20 | 20 | 20 |
| Max. | 50 | 50 | 50 |
| Coloring strength (expressed as crocins) | |||
| 440 nm, on dry matter, min. | 200 | 170 | 120 |
| Artificial colorants | Absent | Absent | Absent |
This table is an extract from a report by ISO 3632.
Safety and toxicity of saffron to humans as food
Although various studies have investigated the toxicity and safety of saffron in animal and human models, a safe dose has not yet been established. In animal experiments using mice, oral administration of saffron extract showed that the LD50 value varied from 4,120 mg to 5 g/kg and was non-toxic (59,60). In addition, following intraperitoneal injection of saffron stigma and tepals extracts in rats, LD50 values were reported to be 1.6 and 6 g/kg, respectively (61). Thus, based on these findings, the saffron extract may be non-toxic in animal experiments.
Although there are no reports on the acceptable daily dietary intake of saffron in humans, the lethal dose is approximately 20 g (62). In addition, it has been reported that saffron is safe or has low toxicity in humans following oral administration (20,63). However, there are no reports on the safety of saffron as a food source. Thus, this section reviews the safety and toxicity of saffron based on several clinical trials and therapeutic applications.
To investigate saffron’s immunomodulatory effects, 100 mg of saffron tablets were administered to healthy adults for six weeks. The results indicated temporary immunomodulatory activities without any side effects (64). Moreover, few studies conducted on healthy humans or pregnant women have reported that saffron at a higher dose from 200 to 400 mg/day (65) or 4 g/day (66), respectively. No adverse effects were noted in any of these studies. Some studies have exposed patients to high saffron doses of 30 mg/day for 16 to 22 weeks for the treatment of Alzheimer’s disease (67,68). No serious side effects were observed. However, few older studies (before 1925) have reported digestive problems and bleeding as a result of 1.2 to 2.0 g of saffron usage. According to another study, adverse effects such as miscarriage, digestive problems, bleeding from the uterus and gastric mucosa, and dizziness were reported after administering more than 10 g of saffron on day 21 (62). However, the adverse events observed in these studies have been inconsistent. In a study where 4 g/day (66) was administered to pregnant women, it is possible that alternatives such as Colchicum autumnale may have been used instead of saffron. Additionally, a significantly higher rate of miscarriage has been reported in pregnant women exposed to saffron (69). The American Herbal Products Association classifies saffron as class 2b, which means that high doses or prolonged consumption during pregnancy should be avoided.
Awareness of food allergy information is important prior to saffron consumption. To the best of our knowledge, there is a single case report of saffron allergy (70). However, according to Lucas et al., the risk of allergy is very low (71). Given the long history of saffron consumption, as well as its use as a spice and to color and flavor food, adverse events due to saffron allergic reactions are rare.
Based on these studies, saffron can be considered safe with low toxicity when consumed in amounts contained in a normal diet, albeit only at specific life stages excluding during pregnancy. Naturally, this is because saffron is normally used in very small quantities as a spice to color and flavor food. However, caution should be exercised when using supplements made from concentrated saffron extracts or purified saffron constituents.
Usage of saffron for cooking
Saffron has a long history of culinary usage worldwide; it is a part of the daily meals in several regions and is an integral part of special dishes, such as those prepared to celebrate marriage and festive occasions such as Christmas and New Year’s. Table 5 presents the main examples of dishes from different countries that use saffron. The spice is frequently used in Iranian (Persian) food, and the country is one of the top exporters of saffron; however, saffron is also an important spice used in Asian and European dishes. Saffron is mainly used as a spice in cooking, and the compound responsible for coloring, taste, and aroma are crocins, picrocrocin, and safranal, respectively. The aroma of saffron is floral, spicy, sweet, herbal, and barky (72). The taste is bitter, and its color in food is a fluorescent yellow-orange. To exploit the advantages of the characteristics of saffron, it is applied to rice, meat, fish, stewed dishes, soup, specialty desserts, confections, teas, and spirits across India, Iran, and Europe. In India, saffron is used in making sweets for customary celebrations.
Table 5
| Region | Country | Name of cuisine | Synopsis |
|---|---|---|---|
| Asia | India | Pulau | Seasoned rice |
| Biryani | Spice-flavored rice | ||
| Korma | Stewed dishes with meat or vegetable | ||
| Kheer (payasam) | Indian pudding from rice | ||
| Shrikhand | Dessert dish made from flavored yogurt | ||
| Ras Malai | Dough in milk cream (sometimes using saffron) | ||
| Kulfi | Traditional Indian ice cream | ||
| Double ka meetha | Bread pudding with spice | ||
| Kuwait | Makboos | Seasoned rice with meat (sometimes using saffron) | |
| Turkey | Zerde | Specialty dessert of rice-pudding | |
| Middle East | Iran | Pilaf | Seasoned rice |
| Polow | Persian seasoned rice dishes | ||
| Sholeh zard | Rice-pudding | ||
| Sohan-e-Qom | Caramelized sugar flavored with saffron and mixed with sesame paste | ||
| Halva | Thick paste made from flour, butter, and saffron | ||
| Jalebi | Pretzel in syrup | ||
| Kesar peda | Specialty dessert dishes made from milk, sugar, and saffron | ||
| Kebab | Roasted meat, fish, or vegetable | ||
| Chai | Tea using various spices with sugar crystals containing saffron | ||
| Saffron tea | Tea (extracting color and flavor of saffron) | ||
| Europe | Italy | Risotto | Cooked rice with a soup containing saffron |
| Arancini | Deep-fried saffron risotto | ||
| Strega | Spirits colored and flavored with saffron | ||
| France | Bouillabaisse | Soup with seafood | |
| Chartreuse | Spirits colored and flavored with saffron | ||
| Izarra | Spirits colored and flavored with saffron | ||
| Spain | Paella | Most popular rice dishes in Spain | |
| Fabada | Stewed dishes with beans | ||
| Germany | Eintopf | Soup with meat, fish, or vegetable | |
| Sweden | Lussekatt | Specialty (Christmas) bun using saffron | |
| Greece | Kozani chicken | Stewed dishes with chicken | |
| Africa | Morocco | Couscous | Made from durum wheat flour |
| Kefta | Seasoned ground beef or lamb | ||
| Mqualli | Fish tagine | ||
| Chermoula | Mixed spice |
Powdered imitations made from turmeric or safflower are sometimes used as substitutes for saffron, given that saffron is quite expensive. The substitutes may reproduce the similar color of saffron, but they cannot reproduce the exotic aroma, exact brilliant color, and saffron-specific flavor, making it an indispensable spice.
Conclusions
Saffron has survived human development and civilization; however, its use has changed with customs and cultural backgrounds. Recently, the production of saffron has decreased due to certain causes, including the COVID-19 pandemic. Nevertheless, a stable supply of high-quality saffron is required because of its cultural significance and historical background. The barriers against the production and supply of saffron are the challenge in cultivation, as well as the low yield and storage conditions. However, the cultivation methods are improving, and new technologies such as tissue culture of Crocus sativus are being developed; these ongoing studies are promising with high expectations. The widespread generalization of these technologies will solve many of the problems associated with saffron, such as safety issues, rendering saffron a highly functional food and safe medicine, and related research fields can further develop. In addition, our review serves as a comprehensive reference for future developments and research in food-related fields dealing with saffron.
Acknowledgments
Funding: This work was supported by the Association for Health Economics Research and Social Insurance and Welfare for financial assistance.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Longhua Chinese Medicine for the series “Multifunctional Saffron”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-1/rc
Peer Review File: Available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-1/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://lcm.amegroups.com/article/view/10.21037/lcm-22-1/coif). The series “Multifunctional Saffron” was commissioned by the editorial office without any funding or sponsorship. YS served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Razinger G, Kozelj G, Gorjup V, et al. Accidental poisoning with autumn crocus (Colchicum autumnale): a case series. Clin Toxicol (Phila) 2021;59:493-9. [Crossref] [PubMed]
- Brncić N, Visković I, Perić R, et al. Accidental plant poisoning with Colchicum autumnale: report of two cases. Croat Med J 2001;42:673-5. [PubMed]
- Sannohe S, Makino Y, Kita T, et al. Colchicine poisoning resulting from accidental ingestion of meadow saffron (Colchicum autumnale). J Forensic Sci 2002;47:1391-6. [Crossref] [PubMed]
- Brvar M, Ploj T, Kozelj G, et al. Case report: fatal poisoning with Colchicum autumnale. Crit Care 2004;8:R56-9. [Crossref] [PubMed]
- Kupper J, Rentsch K, Mittelholzer A, et al. A fatal case of autumn crocus (Colchicum autumnale) poisoning in a heifer: confirmation by mass-spectrometric colchicine detection. J Vet Diagn Invest 2010;22:119-22. [Crossref] [PubMed]
- Galland-Decker C, Charmoy A, Jolliet P, et al. Progressive Organ Failure After Ingestion of Wild Garlic Juice. J Emerg Med 2016;50:55-60. [Crossref] [PubMed]
- Ganeshram R. Saffron: A Global History (Edible) Reaktion Books; 2020.
- Ahrazem O, Rubio-Moraga A, Nebauer SG, et al. Saffron: Its Phytochemistry, Developmental Processes, and Biotechnological Prospects. J Agric Food Chem 2015;63:8751-64. [Crossref] [PubMed]
- Srivastava R, Ahmed H, Dixit RK, et al. Crocus sativus L.: A comprehensive review. Pharmacogn Rev 2010;4:200-8. [Crossref] [PubMed]
- The best way to explore trade data. 2011. Available online: https://oec.world/en. Accessed December 2021.
- Soeda S, Ochiai T, Shimeno H, et al. Pharmacological activities of crocin in saffron. J Nat Med 2007;61:102-11. [Crossref]
- Kafi M, Kamili AN, Husaini AM, et al. An Expensive Spice Saffron (Crocus sativus L.): A Case Study from Kashmir, Iran, and Turkey. Global Perspectives on Underutilized Crops. 2018:109-49.
- Mzabri I, Addi M, Berrichi A. Traditional and modern uses of saffron (Crocus sativus). Cosmetics 2019;6:63. [Crossref]
- Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol 2014;64:65-80. [Crossref] [PubMed]
- José Bagur M, Alonso Salinas GL, Jiménez-Monreal AM, et al. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2017;23:30. [Crossref] [PubMed]
- Leone S, Recinella L, Chiavaroli A, et al. Phytotherapic use of the Crocus sativus L. (Saffron) and its potential applications: A brief overview. Phytother Res 2018;32:2364-75. [Crossref] [PubMed]
- Gresta F, Lombardo GM, Siracusa L, et al. Saffron, an alternative crop for sustainable agricultural systems. A review. Agron Sustain Dev 2008;28:95-112. [Crossref]
- Khanali M, Shahvarooghi Farahani S, Shojaei H, et al. Life cycle environmental impacts of saffron production in Iran. Environ Sci Pollut Res Int 2017;24:4812-21. [Crossref] [PubMed]
- Cadwallader KR. Flavor Chemistry of Saffron. In: Peter Winterhalter RLR. editor. Carotenoid-Derived Aroma Compounds. Flavor Chemistry of Saffron: ACS Publications; 2001:220-39.
- Shakeri M, Hashemi Tayer A, Shakeri H, et al. Toxicity of Saffron Extracts on Cancer and Normal Cells: A Review Article. Asian Pac J Cancer Prev 2020;21:1867-75. [Crossref] [PubMed]
- Sharma T, Kaul S, Dhar MK. Diversity of culturable bacterial endophytes of saffron in Kashmir, India. Springerplus 2015;4:661. [Crossref] [PubMed]
- Samarghandian S, Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacognosy Res 2014;6:99-107. [Crossref] [PubMed]
- Hosseini A, Razavi BM, Hosseinzadeh H. Pharmacokinetic Properties of Saffron and its Active Components. Eur J Drug Metab Pharmacokinet 2018;43:383-90. [Crossref] [PubMed]
- Liakopoulou-Kyriakides M, Kyriakidis DA. Croscus sativus-biological active constitutents. Stud Nat Prod Chem 2002;26:293-312. [Crossref]
- Serrano-Díaz J, Sánchez AM, Martínez-Tomé M, et al. A contribution to nutritional studies on Crocus sativus flowers and their value as food. J Food Compos Anal 2013;31:101-8. [Crossref]
- Velasco-Negueruela A. Universidad Complutense, Madrid. Saffron. In: Peter KV. editor. Handbook of Herbs and Spices. Elsevier; 2001:276-86.
- Ding F, Liu F, Shao W, et al. Efficient Synthesis of Crocins from Crocetin by a Microbial Glycosyltransferase from Bacillus subtilis 168. J Agric Food Chem 2018;66:11701-8. [Crossref] [PubMed]
- Carmona M, Zalacain A, Sánchez AM, et al. Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J Agric Food Chem 2006;54:973-9. [Crossref] [PubMed]
- Sánchez AM, Carmona M, Zalacain A, et al. Rapid determination of crocetin esters and picrocrocin from saffron spice (Crocus sativus L.) using UV-visible spectrophotometry for quality control. J Agric Food Chem 2008;56:3167-75. [Crossref] [PubMed]
- Pfander H, Schurtenberger H. Biosynthesis of C20-carotenoids in Crocus sativus. Phytochemistry 1982;21:1039-42. [Crossref]
- Sánchez AM, Carmona M, Jarén-Galán M, et al. Picrocrocin kinetics in aqueous saffron spice extracts (Crocus sativus L.) upon thermal treatment. J Agric Food Chem 2011;59:249-55. [Crossref] [PubMed]
- Alonso GL, Salinas MR, Esteban-Infantes FJ, et al. Determination of safranal from saffron (Crocus sativus L.) by thermal desorption−gas chromatography. J Agric Food Chem 1996;44:185-8. [Crossref]
- Rabani-Foroutagheh M, Hamidoghli Y, Mohajeri SA. Effect of split foliar fertilisation on the quality and quantity of active constituents in saffron (Crocus sativus L.). J Sci Food Agric 2014;94:1872-8. [Crossref] [PubMed]
- Cardone L, Castronuovo D, Perniola M, et al. The influence of soil physical and chemical properties on saffron (Crocus sativus L.) growth, yield and quality. Agronomy 2020;10:1154. [Crossref]
- Tong Y, Yan Y, Zhu X, et al. Simultaneous quantification of crocetin esters and picrocrocin changes in Chinese saffron by high-performance liquid chromatography-diode array detector during 15 years of storage. Pharmacogn Mag 2015;11:540-5. [Crossref] [PubMed]
- Raina BL, Agarwal SG, Bhatia AK, et al. Changes in pigments and volatiles of saffron (Crocus sativus L.) during processing and storage. J Sci Food Agric 1996;71:27-32. [Crossref]
- Tsimidou M, Biliaderis CG. Kinetic studies of saffron (Crocus sativus L.) quality deterioration. J Agric Food Chem 1997;45:2890-8. [Crossref]
- Jaimand KR, Rezaee MB, Najafi Ashtiany A. Effects of cultivation condition and storage on dried stigmata of Crocus sativus L. stigmata on determination of crocin. J Med Herb 2010;1:29-34.
- Morimoto S, Umezaki Y, Shoyama Y, et al. Post-harvest degradation of carotenoid glucose esters in saffron. Planta Med 1994;60:438-40. [Crossref] [PubMed]
- Jafari SM, Bahrami I, Dehnad D, et al. The influence of nanocellulose coating on saffron quality during storage. Carbohydr Polym 2018;181:536-42. [Crossref] [PubMed]
- Rajabi H, Ghorbani M, Jafari SM, et al. Retention of saffron bioactive components by spray drying encapsulation using maltodextrin, gum Arabic and gelatin as wall materials. Food Hydrocoll 2015;51:327-37. [Crossref]
- Azadi P, Bagheri K, Gholami M, et al. Thin cell layer, a suitable explant for in vitro regeneration. J Agr Sci Tech 2017;19:1429-35.
- Kashtwari M, Wani AA, Dhar MK, et al. Development of an efficient in vitro mutagenesis protocol for genetic improvement of saffron (Crocus sativus L.). Physiol Mol Biol Plants 2018;24:951-62. [Crossref] [PubMed]
- Halim R, Güler B, Gurel A, et al. In vitro callus induction of saffron(Crocus Sativus L.). Int J Innov Res Sci Eng Technol 2018;3:696-700.
- Moshtaghi N. Tissue and cell culture of saffron. Saffron 2020:229-46.
- Sereshti H, Poursorkh Z, Aliakbarzadeh G, et al. Quality control of saffron and evaluation of potential adulteration by means of thin layer chromatography-image analysis and chemometrics methods. Food Control 2018;90:48-57. [Crossref]
- Petrakis EA, Cagliani LR, Polissiou MG, et al. Evaluation of saffron (Crocus sativus L.) adulteration with plant adulterants by (1)H NMR metabolite fingerprinting. Food Chem 2015;173:890-6. [Crossref] [PubMed]
- Guijarro-Díez M, Castro-Puyana M, Crego AL, et al. Detection of saffron adulteration with gardenia extracts through the determination of geniposide by liquid chromatography-mass spectrometry. J Food Compos Anal 2017;55:30-7. [Crossref]
- Marieschi M, Torelli A, Bruni R. Quality control of saffron (Crocus sativus L.): development of SCAR markers for the detection of plant adulterants used as bulking agents. J Agric Food Chem 2012;60:10998-1004. [Crossref] [PubMed]
- Ordoudi SA, de los Mozos Pascual M, Tsimidou MZ. On the quality control of traded saffron by means of transmission Fourier-transform mid-infrared (FT-MIR) spectroscopy and chemometrics. Food Chem 2014;150:414-21. [Crossref] [PubMed]
- Koulakiotis NS, Gikas E, Iatrou G, et al. Quantitation of Crocins and picrocrocin in saffron by HPLC: application to quality control and phytochemical differentiation from other crocus taxa. Planta Med 2015;81:606-12. [Crossref] [PubMed]
- Nenadis N, Heenan S, Tsimidou MZ, et al. Applicability of PTR-MS in the quality control of saffron. Food Chem 2016;196:961-7. [Crossref] [PubMed]
- Sereshti H, Poursorkh Z, Aliakbarzadeh G, et al. An image analysis of TLC patterns for quality control of saffron based on soil salinity effect: A strategy for data (pre)-processing. Food Chem 2018;239:831-9. [Crossref] [PubMed]
- Reddy CN, Bharate SB, Vishwakarma RA, et al. Chemical analysis of saffron by HPLC based crocetin estimation. J Pharm Biomed Anal 2020;181:113094. [Crossref] [PubMed]
- Standardization of ISO 3632-1:2011 Saffron (Crocus sativus L.) — Part 1: Specification. 2nd ed. Geneva, Switzerland: ISO; 2011.
- Hadizadeh F, Mahdavi M, Emami SA, et al. Evaluation of ISO method in saffron qualification. Acta Horticulturae 2007;405-10. [Crossref]
- Valle García-Rodríguez M, Serrano-Díaz J, Tarantilis PA, et al. Determination of saffron quality by high-performance liquid chromatography. J Agric Food Chem 2014;62:8068-74. [Crossref] [PubMed]
- García-Rodríguez MV, López-Córcoles H, Alonso GL, et al. Comparative evaluation of an ISO 3632 method and an HPLC-DAD method for safranal quantity determination in saffron. Food Chem 2017;221:838-43. [Crossref] [PubMed]
- Bahmani M, Rafieian M, Baradaran A, et al. Nephrotoxicity and hepatotoxicity evaluation of Crocus sativus stigmas in neonates of nursing mice. J Nephropathol 2014;3:81-5. [PubMed]
- Ramadan A, Soliman G, Mahmoud SS, et al. Evaluation of the safety and antioxidant activities of Crocus sativus and Propolis ethanolic extracts. J Saudi Chem Soc 2012;16:13-21. [Crossref]
- Karimi G, Taiebi N, Hosseinzadeh H, et al. Evaluation of subacute toxicity of aqueous extract of Crocus sativus L. stigma and petal in rats. J Med Plants 2004;4:29-35.
- Schmidt M, Betti G, Hensel A. Saffron in phytotherapy: pharmacology and clinical uses. Wien Med Wochenschr 2007;157:315-9. [Crossref] [PubMed]
- Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). Exp Biol Med (Maywood) 2002;227:20-5. [Crossref] [PubMed]
- Kianbakht S, Ghazavi A. Immunomodulatory effects of saffron: a randomized double-blind placebo-controlled clinical trial. Phytother Res 2011;25:1801-5. [Crossref] [PubMed]
- Modaghegh MH, Shahabian M, Esmaeili HA, et al. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine 2008;15:1032-7. [Crossref] [PubMed]
- Melnyk JP, Wang S, Marcone MF. Chemical and biological properties of the world's most expensive spice: Saffron. Food Res Int 2010;43:1981-9. [Crossref]
- Akhondzadeh S, Sabet MS, Harirchian MH, et al. Saffron in the treatment of patients with mild to moderate Alzheimer's disease: a 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther 2010;35:581-8. [Crossref] [PubMed]
- Akhondzadeh S, Shafiee Sabet M, Harirchian MH, et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer's disease. Psychopharmacology (Berl) 2010;207:637-43. [Crossref] [PubMed]
- Ajam M, Reyhani T, Roshanravan V, et al. Increased miscarriage rate in female farmers working in saffron fields: a possible effect of saffron toxicity. Asia Pac J Med Toxicol 2014;3:73-5.
- Wüthrich B, Schmid-Grendelmeyer P, Lundberg M. Anaphylaxis to saffron. Allergy 1997;52:476-7. [Crossref] [PubMed]
- Lucas CD, Hallagan JB, Taylor SL. The role of natural color additives in food allergy. Adv Food Nutr Res 2001;43:195-216. [Crossref] [PubMed]
- Narasimhan S, Chand N, Rajalakshmi D. Saffron: quality evaluation by sensory profile and gas chromatography. J Food Qual 1992;15:303-14. [Crossref]
Cite this article as: Fujii S, Morita Y, Ohta T, Uto T, Shoyama Y. Saffron (Crocus sativus L.) as a valuable spice and food product: a narrative review. Longhua Chin Med 2022;5:18.


