Literature review: anti-diabetic potential of some selected edible vegetables in tropical region
Introduction
Diabetes mellitus (DM) is a chronic disease characterised by hyperglycemia, an alarming increase in blood glucose level. It is a metabolic disorder, in which the body produces insufficient or does not respond normally to insulin, a hormone released by β-cells of pancreatic islets, which controls the amount of glucose in the blood (1). Common symptoms experienced by patients with hyperglycemia include polyuria (frequent urination), polydipsia (increased thirst) and polyphagia (excessive hunger). In the absence of insulin, the liver, muscle, and fat cells are unable to absorb glucose from blood to be used as energy. High blood sugar levels may give rise to several complications such as kidney failure, leg amputation, vision loss and nerve damage (neuropathy). DM is a global concern with 463 million adult cases (20–79 years) in 2019, which is expected to reach 700 million by 2045 (2). The number of deaths associated to DM was 1.6 million in 2016 (3), which increased drastically to 4.2 million in 2019 (2). Diabetic patients suffer from either type 1 or type 2 diabetes. Type 1 DM, (juvenile onset or insulin-dependent diabetes) is a condition in which the patient’s body cannot produce insulin due to autoimmune destruction of β-cells. Type 2 DM (adult-onset or non-insulin dependent diabetes), occurs as a result of inadequate release and poor response of the body’s tissues to insulin, causing an impairment of the hormone’s action on the target tissues (4). This causes the pancreatic β-cells to secrete higher amount of insulin, leading to their significant damage. Type 2 DM is considered to be the most common form of diabetes since it constitutes 90% of diabetic patients (5). Gestational diabetes is another type of DM that develops during pregnancy in which the blood glucose levels are above normal range but below the value diagnosed as DM.
The purpose of this review is to gather the information related to the various class of compounds present in commonly consumed vegetables in tropical and subtropical regions. This will also provide an insight into their efficacy and possible role in decreasing blood glucose level. The information provided may be helpful to researchers to explore the potential of these vegetables in combination with synthetic drugs in future research.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/lcm-21-16).
Methods
The literature search was carried out for the period of 2015–2021 from the databases Elsevier, PubMed, and recognized websites. Information regarding identification of phytochemicals was obtained from papers dated before 2015. All the papers included in this manuscript are published papers and are in English Language. The keywords used for search were α-glucosidase, α-amylase, anti-diabetic properties and phytochemicals of selected vegetables. The terms anti-diabetic medications and hyperglycemia inhibitors were also used.
Discussion
Mode of action of anti-diabetic drugs
α-Glucosidase and α-amylase inhibition
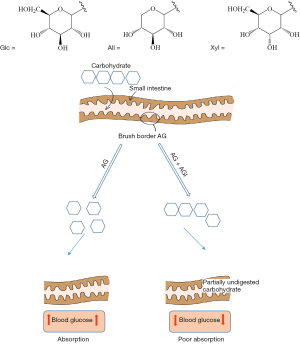
The treatment of DM consists of controlling hyperglycemia either by lowering high blood glucose levels to the normal range or by preventing glucose levels to rise. Inhibition of α-glucosidase and α-amylase enzymes are commonly used to control the blood glucose levels. α-Amylase converts starch by hydrolysis into simple disaccharides and oligosaccharides (6). The unabsorbed oligosaccharides and disaccharides are further broken down into the monosaccharides glucose by α-glucosidase, which is a brush border membrane-bound enzyme in the intestine (7). For diabetic patients, these metabolic processes can cause postprandial hyperglycemia (PPHG) which is defined as the plasma glucose value measured after a meal (8). PPHG is mainly caused by α-glucosidase and is managed by alpha-glucosidase inhibitors (AGIs) (Figure 1). The AGIs compete with oligosaccharides in order to bind to the active sites of α-glucosidase thus delaying complex carbohydrate digestion and glucose absorption by the intestine (9).
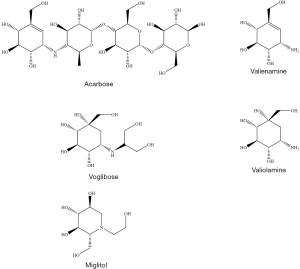
Acarbose, voglibose, and miglitol are the three main AGIs used as therapeutic drugs for the treatment of type 2 DM (Figure 2). Acarbose was first isolated from the fermentation process of the cultures of Actinoplanes utahensis. It was the first approved AGI to be used as a hypoglycemic drug and has contributed more than 25 years of clinical use (10). The drug acarbose contains a valienamine moiety, consisting of a modified pseudotetrasaccharide with a nitrogen atom linking the first and second glucose unit, which accounts for the drug’s high affinity towards active α-glucosidase centres as well as its stability (11). Another compound valiolamine, isolated from Streptomyces hygroscopicus has shown better inhibitory activity against α-glucosidase enzyme compared to valienamine, which led to the synthesis of voglibose (12,13). Diabetic patients with a poor response to their diet and therapeutic exercise are treated with voglibose as a first-line medication. Miglitol, also called glyset, is synthesized from the precursor 1-deoxynojirimycin (14) which can be obtained either from plants extracts (e.g., mulberry tree), microbial cultures of Bacillus and Streptomyces fermentation or chemical synthesis. It is known to delay the conversion of oligosaccharides and complex carbohydrates to glucose and other monosaccharides, and thereby reduces the postprandial rise in blood glucose (15). Acarbose can inhibit both the α-glucosidase and α-amylase enzymes while voglibose and miglitol inhibit only α-glucosidase (10) activity.
ATP-sensitive potassium channel inhibition
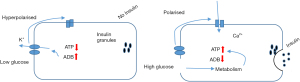
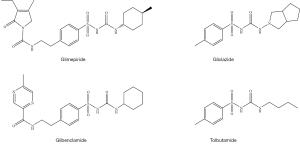
The ATP-sensitive potassium (ATP-K+) channel inhibition promotes insulin secretion to reduce hyperglycemia. When glucose enters the pancreatic β-cells using the glucose transporter 2 (GLUT-2), an increase in glucose concentrations in the cells generates adenosine triphosphates (ATPs) (16). This causes the ATP-K+ channels to close and prevents a leak out of K+ from the channels. An accumulation of K+ inside the β-cell membrane leads to the depolarisation of the membrane’s potential. This change in potential is sensed by the voltage gated calcium channels (VGCC) which opens thereby causing a high concentration of Ca2+ to flow out of the VGCC channel, which results in insulin release by a process called exocytosis (17). As diabetes is known to cause β-cells damage, the patient’s body can no longer exert these processes to secrete insulin. ATP-K+ channel inhibitors such as sulfonylureas, referred to as insulin secretagogues (i.e., insulin releasing agents) are used to block the ATP-K+ channels (18), causing them to close and eventually promotes insulin secretion (Figure 3). Some of the sulfonylureas drugs include glimepiride, gliclazide, glibenclamide and tolbutamide (Figure 4) (19).
Dipeptidyl peptidase IV (DPP-4) inhibition
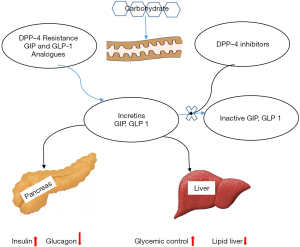
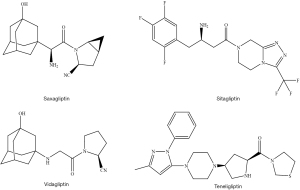
Insulin release is promoted by two metabolic hormones namely glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP). GLP-1 and GIP, commonly known as incretins, are released from enteroendocrine cells of gastrointestinal tract, stomach, and pancreas in response to a meal (Figure 5). Dipeptidyl peptidase IV (DPP-4) enzymes, which are localised in tissues of liver, lung, intestine, and kidney are responsible for the inactivation of incretins (20). By inhibiting the DPP-4 enzymes, an increase in incretins concentration occurs which results into blood glucose lowering effects through stimulation of insulin release and inhibition of glucagon secretion (21). Saxagliptin, sitagliptin, vidagliptin and teneligliptin are common DPP-4 inhibitors drugs (Figure 6).
Peroxisome proliferator-activated receptors-gamma (PPAR-γ) activation
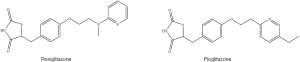
The activation of peroxisome proliferator-activated receptors-gamma (PPAR-γ) leads to insulin sensitisation and improves glucose metabolism. PPAR-γ, present in liver, muscle, and adipose tissue is a subtype of the family PPAR, which is used in gene transcription for regulating glucose and lipid metabolism. Rosiglitazone and pioglitazone, which are in the thiazolidinediones class of drugs (Figure 7) can bind to PPAR-γ receptor to alter the gene transcription related to the glucose and lipid metabolism thereby acting as insulin sensitisers. This causes a change in the gene transcription in adipocytes and modulates fatty acid metabolism causing a decrease in circulating free fatty acids. This decrease in free fatty acids results into an enhanced insulin-signalling in skeletal muscle thus increasing insulin sensitivity (22).
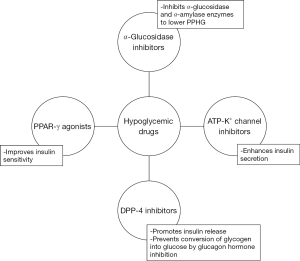
The different hypoglycemic drugs such as α-glucosidase α-amylase inhibitors, ATP-K+ channel inhibitors (sulfonylureas), DPP-4 inhibitors and PPAR-γ activators (thiazolidinediones) used to manage type 2 diabetes along with their properties to treat DM are presented in Figure 8.
These medications can only control the hyperglycemic condition caused by DM but cannot cure the disease completely (23). Apart from their benefits, these drugs also cause a number of side effects including gastrointestinal disturbances such as diarrhea and abdominal bloating (24), enhanced hypoglycemia and weight gain (25). These undesirable side effects and high costs of the synthetic drugs drive the need to explore the natural products as alternate inhibitors, which is also desirable from the pharmacogenetics point of view.
Edible plants
Despite significant advances in management of diabetes with synthetic drug, there has been an increasing attention to find out plant resources as novel anti-diabetic remedies, which are considered to be safe and nontoxic. Several herbs and plants are employed as traditional medicines against major ailments including diabetes (26). The reason for the approach towards plant-based remedies is predominantly due to the presence of a complex array of natural phytochemicals. Some edible plants have been proposed as alternatives or even in combination with the conventional anti-diabetic medications to control blood sugar level. They can help in the development of safer and biologically active natural anti-diabetic drugs with fewer side effects (25). Common edible vegetables belonging to the Cucurbitaceae, Solanaceae, and Amaranthaceae families have been reviewed for their anti-diabetic properties.
Cucurbitaceae family
Cucurbitaceae is a large plant family consisting of 130 genera and 800 species (27). It composes mainly of herbaceous vines or the gourd family with flowering plants, which are collectively called the cucurbits (28). Cucurbitaceous vegetables include bitter gourd, bottle gourd, snake gourd, pumpkin, luffa, and cucumber and they are consumed worldwide for their numerous health benefits and taste. The fruits, seeds, root, and leaves of various cucurbits have shown varying biological properties including α-glucosidase and α-amylase inhibitory activities and show reduction of blood glucose levels in vivo studies.
Bitter gourd
Scientific name: Momordica charantia Linn.
Common names: Bitter gourd, Balsam pear, Bitter melon, Bitter cucumber, African cucumber.
Kingdom: Plantae, Division: Magnoliophyta, Class: Magnoliopsida, Order: Violales, Family: Cucurbitaceae, Genus: Momordica, Species: Charantia.
Bitter gourd (Figure 9) is a tropical plant grown mainly in East Africa, China, India, Caribbean, Central and South America. The plant is a flowering vine with yellow flowers, green leaves, and green fruits turning yellow upon ripening. The fruit has a jagged exterior surface and is fleshy inside with large flat seeds. Momordica charantia is widely consumed as curry, soup, or beverage. Momordica charantia is a world-famous medical vegetable involved in its glucose-lowering effect, improve body stamina, and reduce fatigue (29). Bitter gourd is also consumed as tea and/or in the form of tablets and capsules (30).
The fruit of M. charantia, its extracts and isolated components are known to exert their hypoglycemic activity by promoting the secretion of insulin as well as enhancing its sensitivity. The various extracts of the different parts of the plant including fruits, leaves, pulp, and pericarp are known to exert α-glucosidase and α-amylase inhibitory activities. The % inhibition and IC50 of α-glucosidase and α-amylase inhibitory assays vary greatly depending on the different parts of the plant and solvents used for extraction.
In general, polar fractions of M. charantia have shown better potent inhibitory effects against α-amylase as compared to the non-polar extracts. Fasting and postprandial blood glucose levels in diabetic patients were reduced after the oral intake of the aqueous extract of the fruit pulp (31). Perez et al. (32) reported an α-amylase inhibitory activity of 94% for the acetone extract of the fruit. The protein extracts of the fruits of M. charantia var. charantia (MCC) and M. charantia var. muricata (MCM) also exhibited the α-amylase and α-glucosidase inhibitory activities with a maximum percentage inhibition of 66% to 69% and IC50 values ranging from 0.26 to 0.29 mg/mL. The protein extracts were able to lower the blood glucose level faster than acarbose in Streptozotocin-induced diabetic rats (33). The different extracts of the pulp of M. charantia were found to have the α-glucosidase inhibitory activities in the range of 18–67% (34) while the ethanolic extract of fruits and leaves exhibited inhibition in the range of 16–43% (35) (Table 1). Phytochemicals present in bitter gourd help in managing diabetes as they increase glucose intake and glycogen synthesis (a stored form of glucose) in liver, muscles, and fat cells (38), enhance release of insulin from pancreatic β-cells and promote growth of new insulin-secreting β-cells (39). The main constituents responsible for the anti-diabetic effects are triterpene, protein, steroid, alkaloid, lipid, and phenolic compounds (40,41).
Table 1
| Plant | Part | Extract | α-glucosidase | α-amylase | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Conc (mg/mL) | % inhibition | Conc (mg/mL) | % inhibition | |||||
| Bitter gourd (Momordica charantia) | Pericarp (Chinese) | Hexane | 0.20 | 80.90±6.40 | 0.18 | 75.90±6.50 | (32) | |
| CHCl3 | 54.40±3.00 | 42.72±6.07 | ||||||
| Acetone | 57.90±11.80 | 92.60±1.08 | ||||||
| MeOH | 58.60±5.90 | 77.46±4.97 | ||||||
| MeOH:H2O (8:2) | 50.40±3.40 | 87.51±11.40 | ||||||
| Pericarp (Indian) | Hexane | 91.50±6.90 | 39.54±7.31 | |||||
| CHCl3 | 93.00±8.40 | 71.18±1.60 | ||||||
| Acetone | 63.60±8.40 | 93.67±1.25 | ||||||
| MeOH | 45.00±7.00 | 71.14±1.14 | ||||||
| MeOH:H2O (8:2) | 54.90±0.20 | 37.88±4.44 | ||||||
| Pulp | EtOH | 2.50 | 68.8 | 2.50 | 66.50 | (33) | ||
| Hexane | 10.00 | 19.93±2.31 | – | – | (34) | |||
| CHCl3 | 21.62±0.21 | |||||||
| EtOAc | 66.64±2.94 | |||||||
| MeOH | 18.04±0.47 | |||||||
| Fruit | EtOH | 0.40 | 43.38±3.30 | – | – | (35) | ||
| Leaves | EtOH | 0.40 | 16.52±1.81 | – | – | (35) | ||
| Bottle gourd (Lagenaria siceraria) | Fruits | H2O | – | – | 1.00 | 7.39-14.04 | (6) | |
| Pulp | Hexane | 10.00 | 26.44±3.30 | – | – | (34) | ||
| CHCl3 | 38.61±1.12 | |||||||
| EtOAc | 56.04±1.72 | |||||||
| MeOH | 61.25±2.57 | |||||||
| Seeds | Acetone | 0.30 | 48.19±0.26 | 1.00 | 22.20±1.14 | (36) | ||
| EtOH | 63.23±1.16 | 28.96±0.70 | ||||||
| MeOH | 71.85±0.63 | 16.73±0.47 | ||||||
| H2O | 4.49±0.69 | 10.80±1.14 | ||||||
| Snake gourd (Trichosanthes cucumerina | Pulp | Hexane | 10.00 | 30.87±0.79 | – | – | (34) | |
| CHCl3 | 38.08±0.00 | |||||||
| EtOAc | 61.91±1.96 | |||||||
| MeOH | 12.62±1.75 | |||||||
| Pumpkin (Cucurbita maxima) | Pulp | Hexane | 10.00 | 11.76±1.85 | – | – | (34) | |
| CHCl3 | 10.63±1.06 | |||||||
| EtOAc | 22.11±0.90 | |||||||
| MeOH | 9.93±0.93 | |||||||
| Eggplant (Solanum melongena) | Fruits (green) | Uncooked | 100.0 | 37.85 | – | – | (37) | |
| Cooked | 45.85 | |||||||
| Fruits (white) | Uncooked | 36.35 | ||||||
| Cooked | 40.20 | |||||||
EtOH, Ethanol; MeOH, Methanol; EtOAc, Ethyl Acetate; CHCl3, Chloroform.
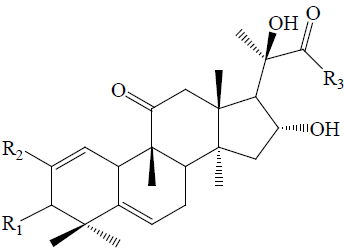
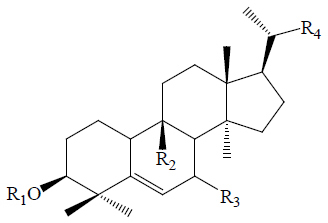
Twelve triterpenes and twenty-seven saponins, one sterol and one flavonoid have been isolated from M. charantia, which showed anti-diabetic activities (42). The triterpenes and saponins are classified as cucurbitacins and oleanane type (Table 2). The cucurbitacins are highly unsaturated tetracyclic terpenes molecules containing the basic ring skeleton 9β-methyl-19-nor-lanosta-5-ene with various oxygenation functionalities throughout its structure (Figure 10). According to their chemical structures, the cucurbitacins or cucurbitane type triterpenes are divided into three subtypes, namely the 5, 19-hemiacetal, normal, and nor-cucurbitanes (43-48,57). The hemiacetal-cucurbitane has two double bonds at C6-C7 and C23-C24, and an oxygen atom linked to C-25, the normal-cucurbitane have double bond at C5-C6 with different substitutions on the carbon skeleton and nor-cucurbitane has got less carbons on the side chain. Cucurbitanes are designated by alphabets A to S according to the presence of various functional groups on rings A and C, diversity in side chain and stereochemical considerations and various aglycone and glycosidic moieties may be present in the molecules (13,54,55,79). The cucurbitanes are responsible for the bitter taste of the fruit. Charantin is a steroidal saponin, which is a mixture of sitosteryl glucoside (C35H60O6) and stigmasteryl glucoside (C35H58O6) in a ratio of 1:1 (Figure 10) (79). Momordicoside, another steroid saponin has been isolated only from bitter gourd (25,44,45,56,57). Other hypoglycemic agents present in M. charantia are polypeptide-p-insulin (hypoglycemic protein), vicine and mormodicin (alkaloid) and lectin (carbohydrate bind protein). Lectin acts on peripheral tissues to reduce blood sugar concentrations and is an appetite suppressant (80).
Table 2
| Cpd. | R1 | R2 | R3 | R4 | R5 | R6 | Vegetable | Biological activity | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 5, 19-hemiacetal cucurbitane subtype triterpenes | |||||||||
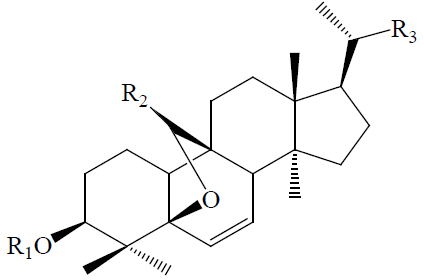 |
|||||||||
| 5β,19-Epoxy-3β,25-dihydroxycucurbita-6,23(E)-diene/(23E)-5β,19-Epoxycucurbita-6,23-dien-3β,25-diol | H | H | 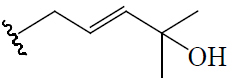 |
BGF | Hypoglycemic (diabetes-induced mice) | (43,44) | |||
| 5β,19-epoxycucurbita-6-ene-23(R),24(S),25-triol | H | H | 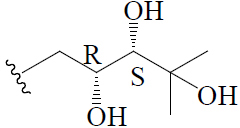 |
BGF | Anti-diabetic | (45) | |||
| (19R,23E)-5β,19-epoxy-19-methoxy-cucurbita-6,23,25-trien-3β-ol | H | OCH3 (R) | 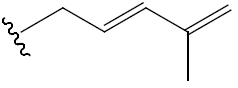 |
BGF | Hypoglycemic (alloxan-induced mice) | (46) | |||
| C2 | 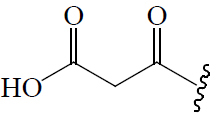 |
OCH3 | 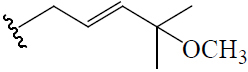 |
BGF | Hypoglycemic (STZ-induced mice) | (47) | |||
| C3 | 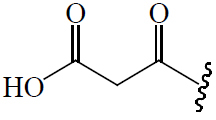 |
=O | 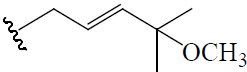 |
BGF | Anti-diabetic | (47) | |||
| Normal-cucurbitane subtype triterpenes | |||||||||
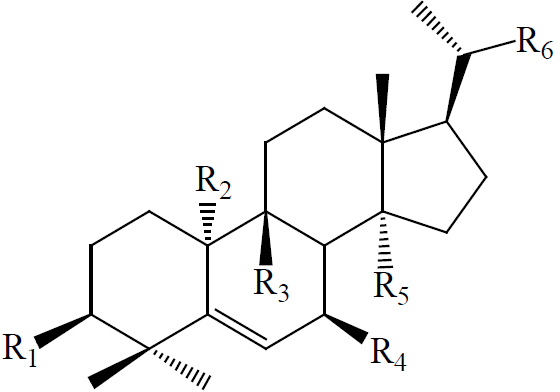 |
|||||||||
| 3β,7β,25-trihydroxycucurbita-5,23(E)-dien-19-al | OH | H | CHO | OH | CH3 | 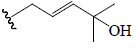 |
BGF | Hypoglycemic (diabetes-induced mice) | (43,44,48,49) |
| α-Amylase | |||||||||
| α-Glucosidase | |||||||||
| 22(S),23(R),24(R),25-tetrahydroxycucurbita-5-ene | OH | H | CH3 | H | CH3 | 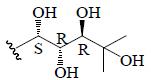 |
BGF | Anti-diabetic | (45) |
| C4 | 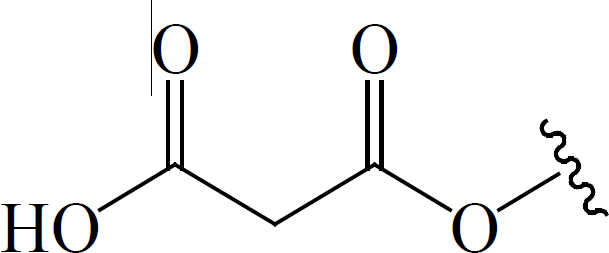 |
H | CHO | OH | CH3 | 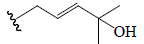 |
BGF | Anti-diabetic | (47) |
| Charantal | OH | CH3 | H | OH | CHO | 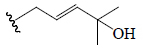 |
BGF | α-Amylase | (48) |
| α-Glucosidase | |||||||||
| Charantoside XI | COOH | H | CHO | OH | CH3 | 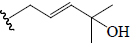 |
BGF | α-Amylase | (48) |
| α-Glucosidase | |||||||||
| Momordicoside K | OH | H | CHO | OGlc | CH3 | 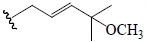 |
BGF | α-Amylase α-Glucosidase | (25) |
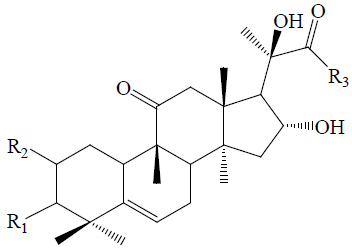 |
|||||||||
| 23,24-dihydrocucurbitacin B | OH | =O | 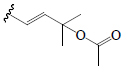 |
SGF | Anti-diabetic | (49) | |||
| Cucurbitacin B | =O | OH | 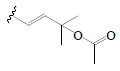 |
BoGF BoGL, BoGR, SGF, PkF (C.pepo) | Hypoglycemic (diabetic mice) | (50-53) | |||
| Cucurbitacin D | =O | OH | 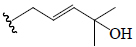 |
BoGF, BoGR, SGF | Anti-diabetic | (50,51) | |||
 |
|||||||||
| 23,24-dihydrocucurbi-tacin E | OH | OH |  |
SGF | Anti-diabetic | (49) | |||
| Cucurbitacin E | =O | OH |  |
BGF, BoGF, SGF, PkF (C. pepo) | Anti-diabetic | (51,54,55) | |||
| 5, 19-hemiacetal cucurbitane saponins | |||||||||
 |
|||||||||
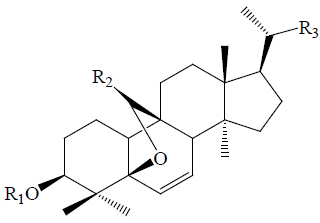
| C1 | All | =O | 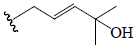 |
BGF | Anti-diabetic | (47) | |||
| Charantoside C | All | =O | 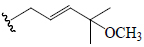 |
BGF | α-Glucosidase | (56) | |||
| Goyaglycoside-b | All | OCH3 | 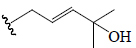 |
BGF | α-Glucosidase | (56) | |||
| Karaviloside XI | All | H | 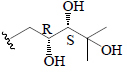 |
BGF | α-Amylase α-Glucosidase |
(25) | |||
| Kuguasaponin G | Glc | H | 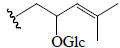 |
BGF | Anti-hyperglycemic | (44) | |||
| Momordicoside F1 | Glc | H | 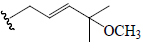 |
BGF | α-Amylase α-Glucosidase |
(25,56) | |||
| Momordicoside F2 | All | H | 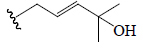 |
BGF | α-Amylase α-Glucosidase |
(25,56) | |||
| Momordicoside G | All | H | 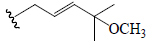 |
BGF | α-Amylase α-Glucosidase |
(25,56) | |||
| Momordicoside I | Glc | H | 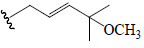 |
BGF | α-Amylase α-Glucosidase |
(25,56) | |||
| Normal-cucurbitane type saponins | |||||||||
 |
|||||||||
| 7β,25-dihydroxycucurbita-5,23(E)-dien-19-al 3-O-β-D-allopyranosyl | All | CHO | OH | 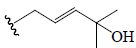 |
BGF | α-Glucosidase | (56) | ||
| Karaviloside III | All | CH3 | OCH3 | 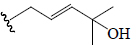 |
BGF | α-Glucosidase | (56) | ||
| Kuguaglycoside G | H | CH3 | OGlc | 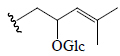 |
BGF | Anti-diabetic | (57) | ||
| Kuguasaponin B | H | CHO | OGlc | 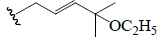 |
BGF | Anti-hyperglycemic | (44) | ||
| Kuguasaponin C | Glc | CHO | OH | 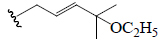 |
BGF | Anti-hyperglycemic | (44) | ||
| Kuguasaponin H | H | CH2OH | OGlc | 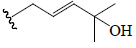 |
BGF | Anti-hyperglycemic | (44) | ||
| Momordicine II | H | CHO | OH | 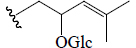 |
BGF | Anti-diabetic | (57) | ||
| Momordicine IV | H | CHO | OGlc | 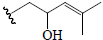 |
BGF | Anti-hyperglycemic | (44) | ||
| Momordicoside A | Glc"( 1-6)-Glc' | CH3 | H | 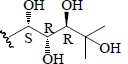 |
BGF | Anti-diabetic, α-Glucosidase |
(45,56) | ||
| Momordicoside C | Glc"( 1-6)-Glc' | CH3 | H | 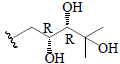 |
BGF | α-Glucosidase | (56) | ||
| Momordicoside M | Glc | CHO | OH | 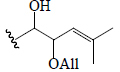 |
BGF | α-Glucosidase | (56) | ||
| Momordicoside S | Glc"( 1-6)-Glc' | CH3 | H | 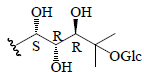 |
BGF | Anti-diabetic | (45) | ||
| Momordicoside T | Xyl'"(1-4)-[Glc''(1-6)]-Glc' | CH3 | H | 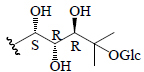 |
BGF | Anti-diabetic | (45) | ||
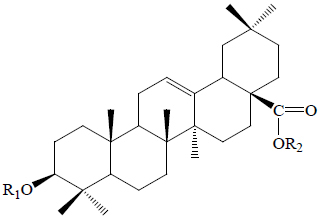
| Oleanane type saponins | |||||||||
 |
|||||||||
| Betavulgaroside II | 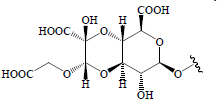 |
H | BeR | Hypoglycemic (oral glucose tolerance test in rats) | (58) | ||||
| Betavulgaroside III | 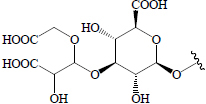 |
Glc | BeR | Hypoglycemic (oral glucose tolerance test in rats) | (58) | ||||
| Betavulgaroside IV | 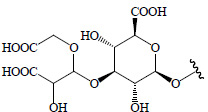 |
H | BeR | Hypoglycemic (oral glucose tolerance test in rats) | (58) | ||||
| Sterols | |||||||||
 |
|||||||||
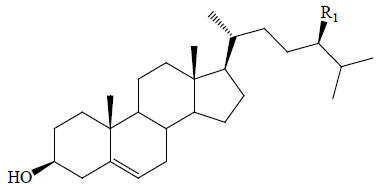
| Campesterol | CH3 | BoGF, PkS | Anti-diabetic | (59,60) | |||||
| Fucosterol | =CHCH3 | BoGF | Anti-diabetic | (60) | |||||
| β-Sitosterol | C2H5 | BGF, BoGF, PkS | Hypoglycemic (diabetic rats) | (59,60-62) | |||||
| Flavonoids | |||||||||
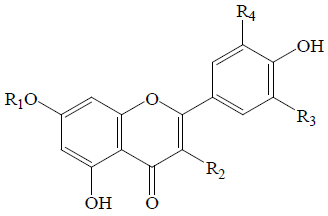 |
|||||||||
| Apigenin | H | H | H | H | EF, EL, EP, BeR | Anti-hyperglycemic (STZ-induced rats), α-Glucosidase | (8,63-67) | ||
| Apigenin-7-glucoside | Glc | H | H | H | BoGF, EF | Anti-diabetic | (63) | ||
| Kaempferol | H | OH | H | H | BGL, BoGF, EF, EL, BeR | α-Glucosidase | (8,59,63,64,66) | ||
| Kaempferol O-sophoroside | H | 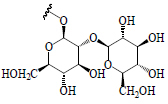 |
H | H | SGF | α-Amylase | (68) | ||
| Luteolin | H | H | H | OH | EF | α-Glucosidase; Anti-diabetic (KK-Ay diabetic and obese mice) | (8,63,69) | ||
| Luteolin-7-O-glucoside | Glc | H | H | OH | BoGF, EF | Anti-diabetic (KK-Ay diabetic and obese mice) | (63,69,70) | ||
| Quercetin | H | OH | H | OH | BGL, EF, EL, BeR | α-Glucosidase | (8,63,64,66) | ||
| Quercetin-3-O-β-D-glucopyranoside (Isoquercetin) | H | OGlc | H | OH | BoGF, BoGPu, EP | α-Glucosidase | (34,59,71) | ||
 |
|||||||||
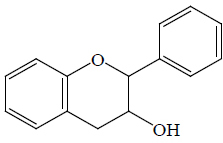
| Catechin | – | BGL, BoGE, BoGM, BoGS, BeR | Hypoglycemic (STZ-induced diabetic rats) | (36,66) | |||||
| Phenolic acids | |||||||||
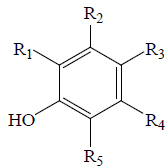 |
|||||||||
| Caffeic acid | H | H | 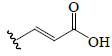 |
H | OH | BGF, BGL, EF, EP, BeR | Anti-hyperglycemic (diabetic mice) α-Amylase; α-Glucosidase | (63,65,66,72) | |
| Chlorogenic acid | OH | H | 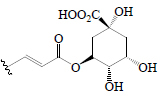 |
H | H | BGL, BoGE, BoGM, BoGS, EF, EP, BeR | α-Amylase; α-Glucosidase | (36,63,65,66,72,73) | |
| Ferulic acid | OCH3 | H | 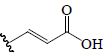 |
H | H | BoGF, EF, BeR | Anti-diabetic (male diabetic albino rats), α-Amylase; α-Glucosidase | (54,63,66,74,75) | |
| Gallic acid | OH | H | COOH | H | OH | BGL, BoGE, BoGS, EP, BeR | α-Amylase; α-Glucosidase | (36,65,66,76) | |
| Protocatechuic acid | H | H | COOH | H | OH | BoGF, BeR | α-Amylase; α-Glucosidase | (66,70,76) | |
| P-coumaric acid | H | H | 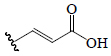 |
H | H | BoGE, BoGM, BoGS, BoGF, BeR | Hypoglycemic (STZ-induced male rats) | (36,66,77) | |
| Vanillic acid | H | H | COOH | H | OCH3 | BoGE, BoGM, BoGS, EP | Hypoglycemic (STZ-induced male rats) | (36,65,78) | |
BGF, Bitter Gourd Fruit; BGL, Bitter Gourd Leaves; BoGF, Bottle Gourd Fruit; BoGPu, Bottle Gourd Pulp; BoGE, Bottle Gourd Epicarp; BoGM, Bottle Gourd Mesocarp; BoGL, Bottle Gourd Leaves; BoGR, Bottle Gourd Roots; BoGS, Bottle Gourd Seeds; SGF, Snake Gourd Fruit; PkF, Pumpkin Fruit; PkS, Pumpkin Seeds; EF, Eggplant Fruit; EP, Eggplant Peel; EL, Eggplant Leaves; BeR, Beetroot Roots.
Bottle gourd
Scientific name: Lagenaria siceraria (Molina) Standley.
Common names: Bottle gourd, Calabash, Lauki.
Kingdom: Plantae, Division: Magnoliophyta, Class: Magnoliopsida, Order: Cucurbitales.
Family: Cucurbitaceae, Genus: Lagenaria, Species: L. siceraria.
Bottle gourd (Figure 11) is a climbing/running vine bearing bottle or oval shaped green raw fruits, becoming brown and hard-shell gourds upon maturation. The interior flesh of the fruit is soft and white with small white/brown seeds. Bottle gourd is a popular and widely cultivated plant due to its various ethnomedicinal properties. The seeds, fruits, and fresh juice of L. siceraria are used as folk medicines to lower blood glucose levels (28,50,70,81,82).
The potency of bottle gourd extracts to inhibit α-glucosidase and α-amylase enzymes suggested its role as anti-diabetic agents. All the extracts of the seed showed better α-glucosidase inhibitory activity compared to the fruit extracts (34,36) (Table 1). The extracts of the fruits showed IC50 values in the range of 0.28–0.48 and 0.2–0.34 mg/mL for α-glucosidase and α-amylase respectively (23). The extract’s efficacy was almost comparable to the drug acarbose, with the aqueous decoction being a better inhibitor of α-glucosidase while ethyl acetate for α-amylase enzyme. Therefore, the fruits and seeds were the most effective parts in inhibiting both enzymes and can be used as alternative treatment for diabetes.
In vivo, the aqueous extract of the fruit showed higher hypoglycemic activity when orally administered to alloxan-induced diabetic mice, exhibiting a decrease of 52% in fasting blood glucose level compared to metformin (49%) (7).
The triterpenes, cucurbitacins B, D, E, G and H, sterols (β-sitosterol, campesterol and fucosterol) and flavonoids (isoquercetin, kaempferol and luteolin-7-O-β-D-glucopyranoside), isolated from bottle gourd, have exhibited anti-diabetic properties (50,52,54,55,59-61,63,69). Isoquercetin (1.0 mg/mL) isolated from the methanolic extract of dried bottle gourd pulps exhibited 83% inhibition of α-glucosidase (34). The flavonoids kaempferol, luteolin and quercetin exhibited higher α-glucosidase activity (IC50: 32, 46 and 15 µM respectively) compared to acarbose (IC50=607 µM) (8).
Snake gourd
Scientific name: Trichosanthes cucumerina.
Common names: Snake gourd, Viper gourd, Snake tomato, Long tomato.
Kingdom: Plantae, Division: Magnoliophyta, Class: Magnoliopsida, Order: Cucurbitales. Family: Cucurbitaceae, Genus: Trichosanthes, Species: T. cucumerina.
Trichosanthes forms the largest genus of the Cucurbitaceae family consisting of over 100 species (51). The genus Trichosanthes originated from tropical area of Southeast Asia and Australia from which snake gourd (Figure 12) is a common vegetable harvested and cooked in various countries, particularly in Malaysian cuisine due to its high water, vitamin C and protein content (83). Snake gourd is an annual climber having slender long green-white fruits with twisted ends (49).
The different extracts of snake gourd pulp were found to inhibit α-glucosidase inhibitory activity in the range of 13–62% inhibition, with the ethyl acetate extract showing the highest activity (34).
Five triterpenes, including cucurbitanes B and D, β-sitosterol and kaempferol-O-sophoroside have been isolated from snake gourd, which have shown hypoglycemic activity (49,51,68,83) (Table 2).
Pumpkin
Scientific name: Cucurbita pepo/Cucurbita maxima/Cucurbita moschata.
Common names: Pumpkin, Kaddu.
Kingdom: Plantae, Division: Magnoliophyta, Class: Magnoliopsida, Order: Cucurbitales. Family: Cucurbitaceae, Genus: Cucurbita, Species: C. pepo/C. maxima/C. moschata.
Pumpkin (Figure 13) is native to Northern Mexico and Southwestern and Eastern USA. The big, ovoid-elliptical shaped orange to green fruit with large leaves are the main characteristics of a pumpkin plant. It has several medicinal and nutritional properties (84). There are different pumpkin species namely Cucurbita pepo, Cucurbita maxima, and Cucurbita moschata, which are known to show hypoglycemic effects (27).
The different extracts of the pulp of Cucurbita maxima showed poor inhibition towards α-glucosidase (34). The ethanolic extracts of pumpkin flesh and seeds (150 mg/kg) showed a significant reduction in the blood glucose level in streptozotocin-induced diabetic mice comparable to the effect induced by metformin (65 mg/kg). The anti-diabetic action could be due to the stimulation of the insulin secretion from pancreatic β-cells (85).
Bioactive phytochemicals present in pumpkin include cucurbitacins B and E, sterols (β-sitosterol and campesterol) and polysaccharides such as pectin (53,62,86).
Solanaceae family
The Solanaceae family, also called the nightshade family, consists of over 300 genera and 3,000 species (87). This family comprises of essential economic plants such as tomato, potato, eggplant, and bell peppers, used as both food and traditional medications (88). Eggplant has been reported to exhibit anti-diabetic properties.
Eggplant
Scientific name: Solanum melongena Linn.
Common names: Eggplant, brinjal, aubergine.
Kingdom: Plantae; Division: Tracheophyta; Class: Magnoliopsida; Order: Solanales; Family: Solanaceae; Genus: Solanum; Species: S. melongena Linn.
Eggplant (Figure 14) consists of 98 different species of which 58 are categorised as Solanum melongena L. Domesticated species of S. melongena L. has round or elongated egg-shaped fruits, which are larger compared to the hard and green fruits of the wild species. The eggplant fruits have a dark purple to black colour and some varieties are even white and green (89). Generally, the eggplant fruit is consumed cooked or eaten raw as snack (37). According to the National Diabetes Education Program of National Institutes of Health, the Mayo Clinic and the American Diabetes Association, an eggplant-based meal can be used to manage type 2 DM due to its low carbohydrate and high fibre content (90,91).
Methanolic fruit extract of eggplant showed high α-glucosidase inhibitory activity (63 µg/mL), while exhibited moderate α-amylase activity (40 µg/mL) (92). The aqueous fruit extracts of the uncooked white and green eggplant varieties exhibited similar IC50 values for the inhibition of both α-glucosidase (0.43 and 0.40) and α-amylase enzymes (0.49 and 0.43) respectively (37) while cooked species exhibited better activity.
Studies using alloxan-induced diabetic rats, showed that various extracts of eggplant leaves can have significant anti-hyperglycemic effect by promoting pancreatic secretion of insulin or reuptake of glucose (93).
Glucosides, phenolic compounds (chlorogenic acid, ferulic acid, gallic acid) and flavonoids (nasunin, delphinidin), carotenoids, alkaloids, saponins melongoside (L, M, N, O, P) and tannins, present in eggplants are known to possess hypoglycemic activity (8,63-65,67,71,73,89,91). Anthocyanin, a sub-group of flavonoids is a natural pigment present in eggplant and they can help in DM through protection of pancreatic β-cells and stimulation of insulin release (94). Chlorogenic acid inhibited α-glucosidase and α-amylase with an IC50 of 9.2 and 9.1 µg/mL respectively (72).
Amaranthaceae family
Amaranthaceae is a family of plants from either southwestern region of the United States, Latin America, or Africa. It comprises of herbs, vines, shrubs, and trees accounting for approximately 800 species and more than 60 genera (95). Common Amaranthaceous crops include beetroot, spinach, and quinoa, among which beetroot has been reported to show hypoglycemic properties.
Beetroot
Scientific name: Beta vulgaris L.
Common names: Beetroot, Red beet, Garden beet, Table beet.
Kingdom: Plantae; Division: Magnoliophyta; Class: Magnoliopsida (Dicotyledons); Order: Caryophyllales; Family: Amaranthacaea; Genus: Beta; Species: Beta vulgaris L.
Beetroot (Figure 15) is a root vegetable obtained from a flowering plant. The edible part of the plant is categorised as a taproot with a flat oblate or globular shape and colour ranging from dark-purplish red to white for a few species (96). It is consumed raw, boiled, or baked food and as juice (97).
The oral administration of aqueous beetroot leaves extract to STZ-induced diabetic rats (adult male albino) gave a reduced blood glucose level (222 mg/dL) compared to the diabetic control group (333 mg/dL) (97).
Beetroot contains a water-soluble red pigment called betalain, known to have anti-diabetic activity. Beetroot is a rich source of vitamins, minerals, phenolics, carotenoids, nitrates, and ascorbic acids (98). Saponins including oleanolic acid and betavulgarosides I, II, III, IV, V, VI, VII, VIII, IX, and X have been isolated from B. vulgaris roots and leaves and these compounds have shown hypoglycemic activity (58,66) (Table 2).
Conclusions
The prevalence of type 2 diabetes is rising alarmingly worldwide. Plant based diet are highly beneficial for preventing, managing, and treating diabetes. The presence of phytochemicals underlies the benefits of a plant-based diet in ameliorating insulin resistance and lowering the blood glucose level including promotion of a healthy body weight, increase in fiber and phytonutrients. The bioactive phytochemicals present in the cucurbitane family can inhibit the breakdown of oligosaccharides and release of D-glucose resulting in less absorption and decrease in hyperglycemia. The different phytochemicals such as triterpenes, proteins, steroids and phenolic isolated from the different vegetables have comparable activity of that of acarbose in context of α-glucosidase and α-amylase inhibition. Further pharmacological, chemical research and clinical trial is required to elucidate the mechanism(s) of the hypoglycemic activity of these edible plants.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Lee Suan Chua) for the series “Bioactive compounds from natural products with antidiabetic potentials” published in Longhua Chinese Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/lcm-21-16
Peer Review File: Available at https://dx.doi.org/10.21037/lcm-21-16
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/lcm-21-16). The series “Bioactive compounds from natural products with antidiabetic potentials” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sun Y, Ma C, Sun H, et al. Metabolism: A Novel Shared Link between Diabetes Mellitus and Alzheimer's Disease. J Diabetes Res 2020;2020:4981814. [Crossref] [PubMed]
- IDF (International Diabetes Federation), 2020. Diabetes facts & figures, accessed 21 February 2021. Available online: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html
- WHO (World Health Organization), 2020. Diabetes, accessed 11 October 2020. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1
- Goyal R, Jialal I. Diabetes Mellitus Type 2. StatPearls [Internet]. Treasure Island, Florida, US: StatPearls Publishing. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513253/
- Chang CI, Chou CH, Liao MH, et al. Bitter melon triterpenes work as insulin sensitizers and insulin substitutes in insulin-resistant cells. J Funct Foods 2015;13:214-24. [Crossref]
- Ahmed D, Ashiq N. In vitro analysis of anti-diabetic and anti-oxidative potential of pedicles of fruit-vegetable bottle gourd. Pak J Pharm Sci 2018;31:2497-501. [PubMed]
- Zeng L, Zhang G, Liao Y, et al. Inhibitory mechanism of morin on α-glucosidase and its anti-glycation properties. Food Funct 2016;7:3953-63. [Crossref] [PubMed]
- Proença C, Freitas M, Ribeiro D, et al. α-Glucosidase inhibition by flavonoids: an in vitro and in silico structure-activity relationship study. J Enzyme Inhib Med Chem 2017;32:1216-28. [Crossref] [PubMed]
- Joshi SR, Standl E, Tong N, et al. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin Pharmacother 2015;16:1959-81. [Crossref] [PubMed]
- Hanefeld M, Mertes G. Treatment: Alpha Glucosidase Inhibitors. In: Huhtaniemi I, Martini L, editors. Encyclopedia of endocrine diseases. Second edition. United States: Academic Press, 2018;1:238-44.
- Tupas GD, Otero MCB, Ebhohimen IE, et al. Anti-diabetic lead compounds and targets for drug development. Phytochemicals as Lead Compounds for New Drug Discovery 2020:127-41.
- Dabhi AS, Bhatt NR, Shah MJ. Voglibose: an alpha glucosidase inhibitor. J Clin Diagn Res 2013;7:3023-7. [PubMed]
- Chen X, Lu Y, Fan Y, Shen Y. Voglibose: An important drug for type 2 diabetes. Validamycin and its derivatives, discovery, chemical synthesis, and biological activity, Elsevier, 2017:237-78.
- Kumar Y, Goyal RK, Thakur AK. Pharmacotherapeutics of miglitol: an α-glucosidase inhibitor. J Anal Pharm 2018;7:617-9.
- Kalra S, Bhutani J. Alpha-glucosidase Inhibitors. In: V. Mohan and R. Unnikrishnan, eds. Diabetology: Type 2 Diabetes Mellitus, Edition 1. Jaypee Publishers. 2014.
- Habtemariam S. Chapter 5 - Current pharmacotherapy options for type 2 diabetes. In: S. Habtemariam, ed. Medicinal foods as potential therapies for type-2 diabetes and associated diseases, the chemical and pharmacological basis of their action. Academic Press, 2019, 89-107.
- Velasco M, Díaz-García CM, Larqué C, et al. Modulation of ionic channels and insulin secretion by drugs and hormones in pancreatic beta cells. Mol Pharmacol 2016;90:341-57. [Crossref] [PubMed]
- Ghadge AA, Kuvalekar AA. Controversy of oral hypoglycemic agents in type 2 diabetes mellitus: Novel move towards combination therapies. Diabetes Metab Syndr 2017;11:S5-S13. [Crossref] [PubMed]
- Diabetes.co.uk, 2019. Sulphonylureas, accessed 28 February 2021. Available online: https://www.diabetes.co.uk/diabetes-medication/sulphonylureas.html
- Ghosh S, Collier A. Section 3 – Management of Diabetes. In: S. Ghosh and A. Collier eds. Churchill’s Pocketbook of Diabetes (Second Edition). Churchill Livingstone, 2012:83-125.
- Ahrén B. DPP-4 inhibition and the path to clinical proof. Front Endocrinol (Lausanne) 2019;10:376. [Crossref] [PubMed]
- Nicholson G, Hall GM. Diabetes mellitus: new drugs for a new epidemic. Br J Anaesth 2011;107:65-73. [Crossref] [PubMed]
- Atique I, Ahmed D, Maqsood M, et al. Solvents for extraction of anti-diabetic, iron chelating, and anti-oxidative properties from bottle gourd fruit. Int J Veg Sci 2017;24:212-26. [Crossref]
- Ibitoye OB, Uwazie JN, Ajiboye TO. Bioactivity-guided isolation of kaempferol as the anti-diabetic principle from Cucumis sativus L. fruits. J Food Biochem 2017;42:e12479. [Crossref]
- Shivanagoudra SR, Perera WH, Perez JL, et al. In vitro and in silico elucidation of antidiabetic and anti-inflammatory activities of bioactive compounds from Momordica charantia L. Bioorg Med Chem 2019;27:3097-109. [Crossref] [PubMed]
- Venkataraman S, Kumaran S, Jayapalan S. Phytochemical constituents and pharmacological activities of Lagenaria siceraria: A comprehensive review. Drug Invent Today 2018;10:1431-6.
- Rolnik A, Olas B. Vegetables from the Cucurbitaceae family and their products: Positive effect on human health. Nutrition 2020;78:110788. [Crossref] [PubMed]
- Kibria G, Karmakar P, Sarwar MS, et al. Comparative study of anti-diabetic effect of some selected plants extract from Cucurbitaceae family in alloxan-induced diabetic mice. Journal of Noakhali Science and Technology University 2017;1:9-18.
- Shah SS, Hussain MI, Aslam MK, et al. Natural products; pharmacological importance of family Cucurbitaceae: a brief review. Mini Rev Med Chem 2014;14:694-705. [Crossref] [PubMed]
- Jia S, Shen M, Zhang F, et al. Recent advances in Momordica charantia: Functional components and biological activities. Int J Mol Sci 2017;18:2555. [Crossref] [PubMed]
- Ahmad N, Hassan MR, Halder H, et al. Effect of Momordica charantia (Karolla) extracts on fasting and postprandial serum glucose levels in NIDDM patients. Bangladesh Med Res Counc Bull 1999;25:11-3. [PubMed]
- Perez JL, Jayaprakasha GK, Patil BS. Metabolite profiling and in vitro biological activities of two commercial bitter melon (Momordica charantia Linn.) cultivars. Food Chem 2019;288:178-86. [Crossref] [PubMed]
- Poovitha S, Parani M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complement Altern Med 2016;16:185. [Crossref] [PubMed]
- Sulaiman SF, Ooi KL. Supriatno. Antioxidant and α-glucosidase inhibitory activities of cucurbit fruit vegetables and identification of active and major constituents from phenolic-rich extracts of Lagenaria siceraria and Sechium edule. J Agric Food Chem 2013;61:10080-90. [Crossref] [PubMed]
- Hwang ES. Comparison of antioxidant capacity and α -glucosidase inhibitory activity between bitter melon (Momordica charantia) fruit and leaf extract. Asian Pac J Trop Biomed 2018;8:189-93. [Crossref]
- Attar UA, Ghane SG. In vitro antioxidant, ant-idiabetic, antiacetylcholine esterase, anticancer activities and RP-HPLC analysis of phenolics from the wild bottle gourd (Lagenaria siceraria (Molina) Standl.). S Afr J Bot 2019;125:360-70. [Crossref]
- Karigidi KO, Ojebode ME, Olaiya CO. Effect of cooking on antioxidant and enzymes activity linked to carbohydrate metabolism and lipid peroxidation of eggplant (solanum melongena). Trop Agri Sci 2018;41:1717-30.
- Sharma S, Katoch V. Anti-diabetic properties of bitter gourd. Int J Econ Plants 2020;7:21-4. [Crossref]
- Chanda R, Samadder A, Banerjee J. Anti-diabetic activity of Momordica Charantia or bitter melon: A Review. Acta Sci Pharm Sci 2019;3:24-30.
- Haque ME, Alam MB, Hossain MS. The efficacy of cucurbitane type triterpenoids, glycosides and phenolic compounds isolated from momordica charantia: a review. Int J Pharm Sci Res 2011;2:1135-46.
- Joseph B, Jini D. Anti-diabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac J Trop Dis 2013;3:93-102. [Crossref]
- Sun L, Zhang X, Dong L, et al. The triterpenoids of the bitter gourd (Momordica Charantia) and their pharmacological activities: A review. J Food Compos Anal 2020;96:103726. [Crossref]
- Harinantenaina L, Tanaka M, Takaoka S, et al. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem Pharm Bull (Tokyo) 2006;54:1017-21. [Crossref] [PubMed]
- Zhang LJ, Liaw CC, Hsiao PC, et al. Cucurbitane-type glycosides from the fruits of Momordica charantia and their hypoglycaemic and cytotoxic activities. J Funct Foods 2014;6:564-74. [Crossref]
- Tan MJ, Ye JM, Turner N, et al. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem Biol 2008;15:263-73. [Crossref] [PubMed]
- Jiang B, Ji M, Liu W, et al. Antidiabetic activities of a cucurbitane-type triterpenoid compound from Momordica charantia in alloxan-induced diabetic mice. Mol Med Rep 2016;14:4865-72. [Crossref] [PubMed]
- Han JH, Tuan NQ, Park MH, et al. Cucurbitane triterpenoids from the fruits of momordica charantia improve insulin sensitivity and glucose homeostasis in streptozotocin-induced diabetic mice. Mol Nutr Food Res 2018;62:e1700769. [Crossref] [PubMed]
- Shivanagoudra SR, Perera WH, Perez JL, et al. Cucurbitane-type compounds from Momordica charantia: Isolation, in vitro antidiabetic, anti-inflammatory activities and in silico modeling approaches. Bioorg Chem 2019;87:31-42. [Crossref] [PubMed]
- Devi ND. Medicinal values of Trichosanthus cucumerina L. (Snake Gourd) – a review. Br J Pharm Res 2017;16:1-10. [Crossref]
- Kumar D, Sharma C, Singh B, et al. Pharmacognostical, phytochemical and pharmacological profile of natural remedy Lagenaria siceraria (Mol.) standly: a review. Br J Pharm Res 2015;7:340-52. [Crossref]
- Suebsakwong P, Chulrik W, Chunglok W, et al. New triterpenoid saponin glycosides from the fruit fibers of Trichosanthes cucumerina L. RSC Adv 2020;10:10461-70. [Crossref]
- Kumbhalkar B, Tamhankar S, Upadhye A. Development of a high-performance thin-layer chromatographic method for quantification of cucurbitacin B in bottle gourd (lagenaria siceraria) for quality control. J Planar Chromat 2015;28:294-9. [Crossref]
- Kim KH, Lee IS, Park JY, et al. Cucurbitacin B induces hypoglycemic effect in diabetic mice by regulation of AMP-activated protein kinase alpha and glucagon-like peptide-1 via bitter taste receptor signaling. Front Pharmacol 2018;9:1071. [Crossref] [PubMed]
- Chanda J, Biswas S, Kar A, et al. Determination of cucurbitacin E in some selected herbs of ayurvedic importance through RP-HPLC. J Ayurveda Integr Med 2020;11:287-93. [Crossref] [PubMed]
- Murtaza M, Khan G, Aftab MF, et al. Cucurbitacin E reduces obesity and related metabolic dysfunction in mice by targeting JAK-STAT5 signaling pathway. PLoS One 2017;12:e0178910. [Crossref] [PubMed]
- Nhiem NX, Kiem PV, Minh CV, et al. alpha-Glucosidase inhibition properties of cucurbitane-type triterpene glycosides from the fruits of Momordica charantia. Chem Pharm Bull (Tokyo) 2010;58:720-4. [Crossref] [PubMed]
- Keller AC, Ma J, Kavalier A, et al. Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro. Phytomedicine 2011;19:32-7. [Crossref] [PubMed]
- Yoshikawa M, Murakami T, Kadoya M, et al. Medicinal foodstuffs. III. Sugar beet. (1): Hypoglycemic oleanolic acid oligoglycosides, betavulgarosides I, II, III, and IV, from the root of Beta vulgaris L. (Chenopodiaceae). Chem Pharm Bull (Tokyo) 1996;44:1212-7. [Crossref] [PubMed]
- Gangwal A, Parmar SK, Sheth NR. Triterpenoid, flavonoids and sterols from lagenaria siceraria fruits. Der Pharm Lett 2010;2:307-17.
- Roopan SM, Devi Rajeswari V, Kalpana VN, et al. Biotechnology and pharmacological evaluation of Indian vegetable crop Lagenaria siceraria: an overview. Appl Microbiol Biotechnol 2016;100:1153-62. [Crossref] [PubMed]
- Ponnulakshmi R, Shyamaladevi B, Vijayalakshmi P, et al. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol Mech Methods 2019;29:276-90. [Crossref] [PubMed]
- Kim MY, Kim EJ, Kim YN, et al. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr Res Pract 2012;6:21-7. [Crossref] [PubMed]
- Alarcón-Flores MI, Romero-González R, Vidal JL, et al. Multiclass determination of phytochemicals in vegetables and fruits by ultra high performance liquid chromatography coupled to tandem mass spectrometry. Food Chem 2013;141:1120-9. [Crossref] [PubMed]
- Piao XM, Chung JW, Lee GA, et al. Variation in antioxidant activity and flavonoid aglycones in eggplant (solanum melongena l.) germplasm. Plant Breed Biotechnol 2014;2:396-403. [Crossref]
- Di Sotto A, Di Giacomo S, Amatore D, et al. A polyphenol rich extract from solanum melongena L. DR2 peel exhibits antioxidant properties and anti-herpes simplex virus type 1 activity in vitro. Molecules 2018;23:2066. [Crossref] [PubMed]
- El-Mesallamy AMD, El-Latif AES, El-Azim MHA, et al. Chemical composition and biological activities of red beetroot (beta vulgaris linnaeus) roots. Egypt J Chem 2019;63:239-46. [Crossref]
- Ren B, Qin W, Wu F, et al. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur J Pharmacol 2016;773:13-23. [Crossref] [PubMed]
- Sultana R, Alashi AM, Islam K, et al. Inhibitory Activities of Polyphenolic Extracts of Bangladeshi Vegetables against α-Amylase, α-Glucosidase, Pancreatic Lipase, Renin, and Angiotensin-Converting Enzyme. Foods 2020;9:844. [Crossref] [PubMed]
- Zang Y, Igarashi K, Li Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A(y) mice. Biosci Biotechnol Biochem 2016;80:1580-6. [Crossref] [PubMed]
- Juee LYM, Naqishbandi AM. Calabash (Lagenaria siceraria) potency to ameliorate hyperglycemia and oxidative stress in diabetes. J Funct Foods 2020;66:103821. [Crossref]
- Singh AP, Wang Y, Olson RM, et al. LC-MS-MS analysis and the antioxidant activity of flavonoids from eggplant skins grown in organic and conventional environments. Food Sci Nutr 2017;8:869-84.
- Oboh G, Agunloye OM, Adefegha SA, et al. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): a comparative study. J Basic Clin Physiol Pharmacol 2015;26:165-70. [Crossref] [PubMed]
- Alarcón-Flores MI, Romero-González R, Vidal JL, et al. Systematic study of the content of phytochemicals in fresh and fresh-cut vegetables. Antioxidants (Basel) 2015;4:345-58. [Crossref] [PubMed]
- Narasimhan A, Chinnaiyan M, Karundevi B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl Physiol Nutr Metab 2015;40:769-81. [Crossref] [PubMed]
- Zheng Y, Tian J, Yang W, et al. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem 2020;317:126346. [Crossref] [PubMed]
- Adefegha SA, Oboh G, Ejakpovi II, et al. Antioxidant and anti-diabetic effects of gallic and protocatechuic acids: a structure–function perspective. Comp Clin Path 2015;24:1579-85. [Crossref]
- Amalan V, Vijayakumar N, Indumathi D, et al. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed Pharmacother 2016;84:230-6. [Crossref] [PubMed]
- Ji G, Sun R, Hu H, et al. Vannilic acid ameliorates hyperglycaemia-induced oxidative stress and inflammation in streptozotocin-induced diabetic rats. J King Saud Univ Sci 2020;32:2905-11. [Crossref]
- Kaushik U, Aeri V, Mir SR. Cucurbitacins - An insight into medicinal leads from nature. Pharmacogn Rev 2015;9:12-8. [Crossref] [PubMed]
- Thakur M, Sharma RK. Bitter Gourd: Health properties and value addition at farm scale. Marumegh 2016;1:17-21.
- Tyagi N, Sharma GN, Shrivastava B. Medicinal value of Lagenaria siceraria: An overview. Int Jour Indi Her Dru 2017;2:36-43.
- Prajapati RP, Kalariya M, Parmar SK, et al. Phytochemical and pharmacological review of Lagenaria sicereria. J Ayurveda Integr Med 2010;1:266-72. [Crossref] [PubMed]
- Sassi A, Khattak MMAK, Taher M. Trichosanthes cucumerina extracts enhance glucose uptake and regulate adiponectin and leptin concentrations in 3T3-L1 adipocytes model. Food Res 2017;2:146-53. [Crossref]
- Perez Gutierrez RM. Review of Cucurbita pepo (Pumpkin) its phytochemistry and pharmacology. Med Chem 2016;6:12-21.
- Marbun N, Sitorus P, Sinaga SM. Anti-diabetic effects of pumpkin (cucurbita moschata durch) flesh and seeds extracts in streptozotocin induced mice. Asian J Pharm Clin Res 2018;11:91-3. [Crossref]
- Behera TK, Sureja AK, Islam S, et al. A.S., 2012. Minor Cucurbits. In: YH Wang, TK Behera and C. Kole, eds. Genetics, Genomics and Breeding of Cucurbits. USA: Science Publishers, 17-60.
- Morris WL, Taylor MA. The Solanaceous Vegetable Crops: Potato, Tomato, Pepper, and Eggplant. In: B. Thomas, BG. Murray and DJ. Mruphy, eds. Encyclopedia of Applied Plant Sciences (Second Edition). Academic Press, 2017.3, 55-58.
- Kandimalla R, Kalita S, Choudhury B, et al. A review on anti-diabetic potential of genus solanum (solanaceae). J Drug Deliv Ther 2015;5:24-7. [Crossref]
- Gürbüz N, Uluişik S, Frary A, et al. Health benefits and bioactive compounds of eggplant. Food Chem 2018;268:602-10. [Crossref] [PubMed]
- Solanke SB, Tawar MG. Phytochemical information and pharmacological activities of eggplant (Solanum Melongena L.): A comprehensive review. EAS J Pharm Pharmacol 2019;1:106-14.
- Kwon YI, Apostolidis E, Shetty K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour Technol 2008;99:2981-8. [Crossref] [PubMed]
- Nwanna EE, Ibukun EO, Oboh G. Inhibitory effects of methanolic extracts of two eggplant species from South-western Nigeria on starch hydrolysing enzymes linked to type-2 diabetes. Afr J Pharm Pharmacol 2013;7:1575-84. [Crossref]
- Jannat K, Ashrafudoulla M, Mizan MFR, et al. In vivo anti-diabetic and lipid lowering activity and In vitro antimicrobial, thrombolytic and cytotoxic activity of different fraction of methanolic extract of Solanum melongena. Clin Pharmacol Biopharm 2018;7:2. [Crossref]
- De Pascual-Teresa S, Sanchez-Ballesta MT. Anthocyanins: from plant to health. Phytochem Rev 2008;7:281-99. [Crossref]
- Basu S, Zandi P, Sengupta R, et al. Amaranthaceae: The pigweed family. The Encyclopedia of Earth. 2014.
- Ninfali P, Angelino D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013;89:188-99. [Crossref] [PubMed]
- Ali MMA. Protective effects of beetroot on streptozotocin induced diabetes in adult male albino rats. Bulletin of Egyptian Society for Physiological Sciences 2021;41:270-82. [Crossref]
- Chhikara N, Kushwaha K, Sharma P, et al. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem 2019;272:192-200. [Crossref] [PubMed]
Cite this article as: Jhaumeer Laulloo SB, Bhowon MG, Jalloo Y. Literature review: anti-diabetic potential of some selected edible vegetables in tropical region. Longhua Chin Med 2021;4:33.