Effectiveness of Xuebijing injection for sepsis: an overview of systematic reviews and meta-analyses
Sepsis is a devastating condition caused by dysregulated host response to infection which leads to organ failure and death (1). In 2017, an estimated 49 million incident cases of sepsis were recorded worldwide among which more than 40% were children younger than 5 years, and 11 million sepsis-related deaths were reported, representing about 1/5 of all global deaths (2). Sepsis not only imposes a high burden on hospitalized patients (3), but also affects their quality of life, because half of the discharged patients are still not completely recovered (4,5). It has become a global public health concern due to its high mortality and morbidity and substantial economic burden (6). Moreover, with aging of the population, the presence of more people suffering from chronic diseases or on immunosuppressive medications, and the increase in invasive procedures, the incidence of sepsis will continue to increase (7,8).
Sepsis is a medical emergency and should be treated as quickly and efficiently as possible once it has been identified. Each hour of delay in treatment over the ensuing 6 hours was associated with an average decrease in survival of 7.6% (9). Current managements for the treatment of sepsis are fluid resuscitation, source control, antibiotic therapy and organ support therapy (7). Despite modern advances in critical care, most of the managements for sepsis are largely supportive but not specific. In other words, sepsis is a common illness associated with substantial lethality but has no specific treatment. Sepsis thus still remains a scientific and clinical challenge. There is an urgent need to find new drugs and therapies for sepsis. Recently, Xuebijing injection (XBJ) originating from complementary and alternative medicines has been developed to treat sepsis.
XBJ consists of the following five Chinese herbs: Hong Hua (Carthamus tinctorius L.), Chi Shao (Paeonia lactiflora Pall.), Chuan Xiong (Ligusticum chuanxiong Hort.), Dan Shen (Salvia miltiorrhiza Bge.) and Dang Gui [Angelica sinensis (Oliv.) Diels] (10). Its main components are hydroxysafflor yellow A, paeoniflorin oxide, Ligusticum chuanxiong lactone I and paeoniflorin, etc. (11). And it has been approved for the treatment of sepsis in China since 2004 and has been widely used as an add-on treatment for sepsis or septic shock with few side effects (10). XBJ has many pharmacological mechanisms including anti-inflammatory, anti-coagulation, immune regulation, vascular endothelial protection, anti-oxidative stress and others (12).
Currently, a number of randomized controlled trials (RCTs) have been conducted (13-15), and subsequently increasing numbers of systematic reviews (SRs) and meta-analyses (MAs) (10,16-18), have arisen to evaluate the effectiveness of XBJ for sepsis. However, most of the SRs/MAs reported that the evidence supporting the effectiveness of XBJ is insufficient, and those SRs/MAs reported varied and heterogeneous results and low methodological quality, making it difficult to draw a comprehensive conclusion on the effectiveness of XBJ on sepsis. Furthermore, no critically designed overview has been performed to assess the reporting and methodological quality of the published SRs/MAs so far.
Therefore, this overview aims to evaluate the methodological quality and evidence quality of extant SRs/MAs and to provide comprehensive evidence to identify whether XBJ is an effective treatment for sepsis. We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/lcm-21-13).
Methods
This overview of SRs/MAs has been conducted according to the methodological recommendations by the Cochrane Collaboration (19), and has been registered with INPLASY (20) (registration no. INPLASY2020120126).
Search strategy
The four international electronic databases of PubMed, Embase, Cochrane Library, and Web of Science and four Chinese electronic databases of the China National Knowledge Infrastructure Database (CNKI), WANFANG DATA, Chongqing VIP (CQVIP) and Chinese Biomedical Literature Database (CBM) were searched from their inception to September 30, 2020 without language restriction. The basic search strategies were as follows: (“sepsis” OR “severe sepsis” OR “septic shock”) AND (“xuebijing” OR “xue bi jing” OR “XBJ”) AND (“systematic review” OR “meta-analysis”). Meanwhile, we also searched conference abstracts and the reference lists of all retrieved articles to avoid missing relevant SRs/MAs. Details of the literature search strategy are shown in Appendix 1.
Literature screening
The database in Endnote software (version X9) were created. Duplicates were eliminated first, then titles and abstracts were read for the preliminary screening. Whenever we could not definitively exclude articles based on the titles and abstracts, full texts were downloaded and filtered again until all SRs/MAs were confirmed. Literatures were screened by two investigators (YL Shi and CT Chen), and any inconsistencies were discussed with the other two investigators (YD Xu and YJ Chen).
The inclusion criteria were: (I) patients were diagnosed with sepsis; (II) the intervention groups were XBJ plus routine treatment (RT); (III) the control groups were RT alone, and RT comprises fluid resuscitation, source control, antibiotic therapy and organ support therapy (7); (IV) at least one outcome followed was measured: 28-day mortality, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores (the higher the score, the more frequent the need for monitoring and treatment), infection [measured by white blood cells (WBC) or procalcitonin (PCT) or C-reactive protein (CRP)], or coagulation function [measured by platelet (PLT) or activated partial thromboplastin time (APTT) or prothrombin time (PT)]; 5) SRs/MAs of RCTs.
The exclusion criteria were: (I) interventions which combined XBJ with other drugs that affect the efficacy judgment (e.g., ulinastatin); (II) protocols of SRs/MAs, commentaries; (III) studies that published in abstracts forms for which full texts were unavailable; (IV) duplicate reports of the same study.
Data extraction
One researcher (YL Shi) extracted the following basic information: first author, publication date, number of included trials and participants, interventions, outcomes reported, quality assessment tools, and overall conclusions. Another researcher (CT Chen) checked it against the original, and if there was any discrepancy, the original text was referred and the data will be revised accordingly.
Methodological quality
The methodological quality of the included reviews was assessed by researchers (YL Shi and CT Chen) according to the Assessment of Multiple Systematic Reviews 2 (AMSTAR 2) tool (21), which contains 16 items with 7 items (2,4,7,9,11,13,15) were considered crucial domains that critically affect the validity of a review and its conclusions. Any inconsistencies were resolved via discussion with the other two authors (YD Xu and YJ Chen).
Each item was evaluated as “methodological requirements met”, “methodological requirements partly met” or “methodological requirements not met”. Overall confidence in the results of the reviews was rated “high” (none or one non-critical weakness), “moderate” (>1 non-critical weakness but no critical flaws), “low” (1 critical ± non-critical weakness), and “critically low” (>1 critical flaw ± non-critically weakness).
Evidence quality
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system (22) was applied to evaluate the evidence quality of the concerned outcomes (28-day mortality, APACHE II scores, WBC, PCT, CRP, PLT, APTT, PT). For each outcome, we awarded a high grade to begin with as these were RCTs and downgraded if there were problems relating to risk of bias, inconsistency, indirectness, imprecision, or publication bias. We classified evidence quality as high, moderate, low, or very low. The two researchers (YL Shi and CT Chen) independently assessed the quality of evidences and resolved disputes through discussions with the other two researchers (YD Xu and YJ Chen).
Results
Literature selection
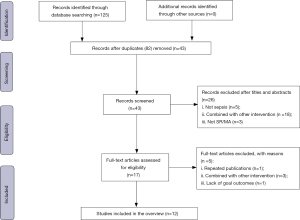
A total of 125 articles were identified from the database. Through strict screening, 12 reviews (10,16-18,23-30) were finally included in this overview. The flow diagram of literature screening is shown in Figure 1. The characteristics of excluded studies are shown in Appendix 2.
Study characteristics
Twelve SRs/MAs were included for the overview, with number of RCTs from 8 to 49 and of participants from 399 to 3,884. All of the 12 reviews conducted MAs. Two tools of quality assessment were employed in MAs, including Cochrane Handbook for Systematic Reviews of Interventions (10,18,26-28,30) and Jadad scale (10,16,17,23-25,29). The characteristics of the included 12 reviews are demonstrated in Table 1.
Table 1
| Study | N [n] | Treatment intervention [n] | Control intervention [n] | Outcome measures | Quality assessment tool | Main conclusion |
|---|---|---|---|---|---|---|
| Li |
16 [1,144] | XBJ + RT [581] | RT [563] | 28-day mortality, mortality during treatment, APACHE II scores, WBC counts, body temperature, adverse events | Jadad Scale; Cochrane Handbook for Systematic Reviews of Interventions | Supplementation with XBJ in addition to RT may improve the 28-day mortality rate, APACHE II scores, WBC count and body temperature of sepsis patients without serious adverse events, but it may not reduce mortality during treatment, revealing a specific, remote effect of traditional Chinese medicine |
| Shi |
49 [3,884] | XBJ + RT [2,023] | RT [1,861] | 28-day mortality, APACHE II score, PCT, WBC, CRP, NEU, temperature | Jadad Scale | XBJ could be a credible alternative for patients with sepsis and shorten the APACHE II score, WBC, CRP, NEU, temperature, and 28-day mortality. However, a need remains for larger samples, data from multi-centers, and high-quality studies to confirm the clinical efficacy of XBJ in the treatment of sepsis patients |
| Hou |
14 [867] | XBJ + RT [438] | RT [429] | PLT, APTT, PT, TT, FIB | Jadad Scale | XBJ injection may improve coagulopathy in patients with sepsis. High-quality and large sample clinical trials are needed for confirmation |
| Zhang |
15 [930] | XBJ + RT [468] | RT [462] | 28-day mortality, APACHE II score, PLT, DD, TNF-α, vWF, sE-selectin, ESM-1, sTM | Cochrane Handbook for Systematic Reviews of Interventions | XBJ injection can not only effectively reduce the release of inflammatory factors, thereby improving vascular endothelial injury, reducing coagulation disorders and blocking coagulation-inflammation network; it can also increase the level of platelets, thereby repairing injured vascular endothelial cells, which has a certain value to reduce the condition of sepsis and improve the prognosis. It also provides some basis for the treatment of sepsis secondary to novel coronavirus pneumonia |
| Wu |
14 [938] | XBJ + RT [475] | RT [463] | 28-day mortality, APACHE II score, WBC, CRP | Cochrane Handbook for Systematic Reviews of Interventions | XBJ injection can improve the clinical symptoms, significantly reduce the mortality, and has high clinical application value |
| Zhou |
8 [399] | XBJ + RT [202] | RT [197] | 7-day mortality, 14-day mortality, 28-day mortality | Jadad Scale | Supplementation with XBJ in addition to RT may reduce relative average mortality rate, the available evidence was sufficient to support XBJ being used as an adjunctive therapy for septic shock patients |
| Xu, 2016 | 8 [502] | XBJ + RT [251] | RT [251] | 28-day mortality, APACHE II score, HLA-DR, CD4+ T lymphocytes, CD8+ T lymphocytes, CD4+/CD8+ T lymphocyte ratio | Cochrane Handbook for Systematic Reviews of Interventions | The available evidence showed that XBJ injection could improve immune dysfunction in sepsis |
| Li |
11 [803] | XBJ + RT [406] | RT [397] | Mortality during observation period, APACHE II score, WBC, PCT, CRP, ORR | Cochrane Handbook for Systematic Reviews of Interventions | The existing clinical evidence shows that the addition of XBJ injection on the basis of routine treatment can improve the clinical efficacy of septic shock |
| Sun |
13 [1,468] | XBJ + RT [756] | RT [712] | 28-day mortality, APACHE II score, PLT, PT, APTT, TT, FIB, DD | Cochrane Handbook for Systematic Reviews of Interventions | XBJ injection can improve blood coagulation in patients with sepsis and also improve the prognosis of patients |
| Xu |
18 [1,172] | XBJ + RT [596] | RT [576] | 28-day mortality | Jadad scale | Supplementation with XBJ in addition to RT can effectively improve the 28-day mortality rate of patients with sepsis |
| Li |
13 [1,280] | XBJ + RT [733] | RT [547] | 28-day mortality, APACHE II score, PCT, WBC, CRP, PLT, PT, APTT | Jadad scale | XBJ injection has certain effect in improving the inflammatory response, coagulation function in patients with sepsis, reducing mortality and improving the APACHE II scores |
| Sun |
18 [1,080] | XBJ + RT [539] | RT [541] | WBC, PLT, TNF-α | Jadad scale | Supplementation with XBJ in addition to RT can reduce WBC count and TNF-α and increase the level of platelets compared to RT alone |
N, number of included randomized controlled trials; n, number of participants; XBJ, Xuebijing injection; RT, routine treatment; APACHE II score, Acute Physiology and Chronic Health Evaluation II score; WBC, white blood cells; PCT, procalcitonin; CRP, C-reactive protein; NEU, neutrophil; PLT, platelet; APTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time; FIB, fibrinogen; DD, D-dimer; TNF-α, tumor necrosis factor-α; vWF, von Willebrand Factor; ESM, endothelial cell specific molecule; sTM, soluble thrombomodulin; HLA-DR, human leukocyte antigen-DR; ORR, overall response rate.
Methodological quality evaluation of included MAs
According to the AMSTAR 2 results, all included MAs were rated as critically low quality. The main causes influencing the methodological quality of reviews are item 2 (none of the included reviews contain an explicit statement that the review methods were established prior to the conduct of the review), item 3 (none of the included reviews explain their selection of the study designs for inclusion in the review), item 7 (none of the included reviews provide a list of excluded studies) and item 10 (none of the included reviews report on the sources of funding for the studies included in the review). The methodological quality evaluations of included reviews are presented in Figure 2.

Evidence quality evaluation of outcomes
We evaluated the evidence quality for the 45 outcomes according to the GRADE system. Only 1 (2.2%) outcome was rated as high-quality evidence, 10 (22.2%) were rated as moderate quality, 28 (62.2%) were rated as low quality, and 6 (13.3%) were rated as very low quality.
The main factor for downgrading was the risk of bias (the included studies of all outcomes designed with a bias in random sequence generation, allocation concealment, blinding, or incomplete outcome data) and only 2 (4.4%) outcomes did not present this issue. Of 45 outcomes, evidence quality was downgraded for 17 (37.8%) due to imprecision, and for 24 (53.3%) due to inconsistency. There was no publication bias and indirectness in all outcomes. The details of the evidence quality are shown in Table 2.
Table 2
| Included studies | Intervention | Control | Outcomes | Effect estimate (95% CI) | N [n] | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Quality of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Li 2018 | XBJ + RT | RT | 28-day mortality | RR 0.62 (0.51, 0.76) | 13 [934] | Seriousa | Not serious | Not serious | Not serious | Not serious | M |
| APACHE II scores | MD −3.53 (−4.49, −2.54) | 12 [792] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| WBC, 400 mL/d | MD −8.00 (−10.18, −5.82) | 1 [40] | Seriousa | NA | Not serious | Seriousc | Not serious | L | |||
| WBC, 200 mL/d | MD −2.38 (−5.01, 0.25) | 3 [230] | Seriousa | Seriousb | Not serious | Seriousc | Not serious | VL | |||
| WBC, 100 mL/d | MD −2.88 (−3.79, −1.96) | 3 [242] | Seriousa | Not serious | Not serious | Seriousc | Not serious | L | |||
| Shi 2017 | XBJ + RT | RT | 28-day mortality | RR 0.51 (0.44, 0.59) | 32 [2,315] | Seriousa | Not serious | Not serious | Not serious | Not serious | M |
| APACHE II scores | WMD −3.70 (−4.31, −3.09) | 34 [2,838] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| WBC | WMD −1.48 (−2.03, −0.94) | 27 [1,678] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| PCT | WMD −1.26 (−1.63, −0.88) | 19 [1,497] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| CRP | WMD −24.38 (−30.49, −18.26) | 23 [1,643] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| Hou 2015 | XBJ + RT | RT | PLT | MD 42.14 (22.42, 61.86) | 12 [675] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L |
| APTT | MD −4.81 (−7.86, −1.76) | 14 [867] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| PT | MD −2.33 (−4.15, −0.51) | 14 [867] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| Zhang 2020 | XBJ + RT | RT | 28-day mortality | OR 0.52 (0.38, 0.71) | 8 [518] | Seriousa | Not serious | Not serious | Not serious | Not serious | M |
| APACHE II scores | WMD −2.65 (−3.23, −2.08) | 6 [466] | Not serious | Not serious | Not serious | Not serious | Not serious | H | |||
| PLT | WMD 30.78 (25.65, 35.92) | 5 [332] | Not serious | Not serious | Not serious | Seriousc | Not serious | M | |||
| Wu 2020 | XBJ + RT | RT | 28-day mortality | RR 0.52 (0.40, 0.67) | 8 [497] | Seriousa | Not serious | Not serious | Not serious | Not serious | M |
| APACHE II scores | MD −5.48 (−7.52, −3.43) | 9 [574] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| WBC | MD −2.26 (−3.35, −1.17) | 10 [726] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| CRP | MD −37.43 (−56.70, −18.16) | 7 [509] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| Zhou 2016 | XBJ + RT | RT | 28-day mortality | RR 0.61 (0.41, 0.90) | 4 [200] | Seriousa | Not serious | Not serious | Seriousc | Not serious | L |
| Xu 2016 | XBJ + RT | RT | 28-day mortality | – | 1 [21] | Seriousa | NA | Not serious | Seriousc | Not serious | L |
| APACHE II scores | MD −2.83 (−4.82, −0.84) | 4 [177] | Seriousa | Seriousb | Not serious | Seriousc | Not serious | VL | |||
| Li 2016 | XBJ + RT | RT | 28-day mortality | OR 0.33 (0.20, 0.57) | 7 [372] | Seriousa | Not serious | Not serious | Not serious | Not serious | M |
| APACHE II scores | MD −4.01 (−4.88, −3.13) | 5 [273] | Seriousa | Not serious | Not serious | Seriousc | Not serious | L | |||
| WBC | MD −4.31 (−6.73, −1.89) | 2 [96] | Seriousa | Not serious | Not serious | Seriousc | Not serious | L | |||
| CRP | MD −2.82 (−3.74, −1.91) | 2 [312] | Seriousa | Not serious | Not serious | Seriousc | Not serious | L | |||
| PCT | MD −1.42 (−1.90, −0.95) | 2 [312] | Seriousa | Not serious | Not serious | Seriousc | Not serious | L | |||
| Sun 2015 | XBJ + RT | RT | 28-day mortality | OR 0.74 (0.56, 0.97) | 5 [1,049] | Seriousa | Not serious | Not serious | Not serious | Not serious | M |
| APACHE II scores | MD −3.30 (−5.38, −1.21) | 5 [358] | Seriousa | Seriousb | Not serious | Seriousc | Not serious | VL | |||
| PLT | MD 51.39 (45.55, 57.24) | 12 [737] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| APTT | MD −3.88 (−4.77, −2.98) | 12 [737] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| PT | MD −1.87 (−2.59, −1.16) | 12 [737] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| Xu 2014 | XBJ + RT | RT | 28-day mortality, 100 mL/d | RR 1.20 (1.11, 1.29) | 13 [850] | Seriousa | Not serious | Not serious | Not serious | Not serious | M |
| 28-day mortality, 200 mL/d | RR 1.24 (1.00, 1.54) | 4 [194] | Seriousa | Not serious | Not serious | Seriousc | Not serious | L | |||
| Li 2013 | XBJ + RT | RT | 28-day mortality | OR 0.39 (0.27, 0.58) | 8 [562] | Seriousa | Not serious | Not serious | Not serious | Not serious | M |
| APACHE II scores | WMD −3.43 (−4.72, −2.15) | 6 [375] | Seriousa | Seriousb | Not serious | Seriousc | Not serious | VL | |||
| WBC | WMD −2.94 (−3.49, −2.38) | 3 [274] | Seriousa | Not serious | Not serious | Seriousc | Not serious | L | |||
| CRP | WMD −9.81 (−21.51, 1.90) | 4 [306] | Seriousa | Seriousb | Not serious | Seriousc | Not serious | VL | |||
| PCT | WMD −7.25 (−16.58, 2.08) | 2 [126] | Seriousa | Seriousb | Not serious | Seriousc | Not serious | VL | |||
| PLT | WMD 40.63 (14.09, 67.16) | 8 [519] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| APTT | WMD −4.59 (−6.69, −3.50) | 8 [519] | Seriousa | Not serious | Not serious | Not serious | Not serious | M | |||
| PT | WMD −1.72 (−2.38, −1.06) | 8 [519] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L | |||
| Sun 2012 | XBJ + RT | RT | WBC | WMD −1.87 (−2.92, −0.81) | 9 [545] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L |
| PLT | WMD 6.58 (4.01, 9.16) | 7 [436] | Seriousa | Seriousb | Not serious | Not serious | Not serious | L |
a, the design of the experiment with a large bias in random, allocation concealment, blinding, or incomplete outcome data; b, the confidence interval overlaps less, the heterogeneity test P is very small, and the I2 is larger; c, the simple size is small, and the confidence interval is wide. CI, confidence interval; XBJ, Xuebijing injection; RT, routine treatment; RR, relative risk; MD, mean difference; WMD, weighted MD; WBC, white blood cells; APACHE II score, Acute Physiology and Chronic Health Evaluation II score; PCT, procalcitonin; CRP, C-reactive protein; PLT, platelet; APTT, activated partial thromboplastin time; PT, prothrombin time; OR, odds ratios; H, high; M, moderate; L, low; VL, very low; NA, not applicable.
Effectiveness of XBJ for sepsis
Twenty-eight-day mortality
A total of ten reviews (10,17,18,24-30) analyzed the 28-day mortality of XBJ for sepsis, with RCTs from 1 to 32 and participants from 21 to 2,315. Nine reviews suggested that upon comparison of the effects of XBJ plus RT vs. RT alone, the combined treatment had a significantly greater effect. However, one review (25) pointed out that XBJ could effectively reduce 28-day mortality at a dose of 100 mL/d, but not necessarily at 200 mL/d. According to the GRADE system, the quality of evidences for 28-day mortality was low to moderate.
APACHE II scores
Eight reviews (10,17,18,24,26-28,30), with number of RCTs from 4 to 34 and of participants from 177 to 2,838 compared the effects of XBJ plus RT treatment vs. RT treatment alone using the APACHE II scores, and the results revealed that the combined treatment had a better effect than RT alone. Only one (30) of them was rated high-quality evidence and the others were very low to low.
WBC
Six reviews (10,17,18,23,24,27) reported the effectiveness of XBJ for sepsis on WBC, among which 1–27 RCTs and 40–1,678 participants were included. One review (10) reported that XBJ combined with RT at doses of 400 and 100 mL/d worked better in improving WBC count than RT alone, but not at a dose of 200 mL/d group. The quality of evidence for WBC was very low to low.
CRP
Four MAs (17,18,24,27) reported the pooled results of XBJ plus RT vs. RT alone, and the number of RCTs included in the MAs ranged from 2 to 23 with participants from 306 to 1,643. Three MAs (17,18,27) with low-grade evidence indicated that combined treatment was superior to RT alone, and the reduction of the CRP was significantly higher in the XBJ plus RT group. However, one review (24) which was rated very low grade found no significant difference in CRP between two groups.
PCT
Three reviews (17,24,27) used the PCT level to compare the effects of XBJ plus RT vs. RT alone, and two reviews (17,27) showed that the combined treatment could significantly reduce the PCT more than RT alone, which had low quality of evidence. One MA (24), rated very low-grade evidence, analyzed 2 RCTs with 126 participants and came to the conclusion that there was no significant difference in PCT between the two groups.
PLT
Five reviews (16,23,24,26,30) used the PLT to access the effectiveness of XBJ for sepsis, and 5–12 RCTs and 332–737 participants were included. All of them indicated that compared with the control group, PLT counts markedly increased in the treatment group. The quality of evidence for PLT was low to moderate.
APTT and PT
The APTT and PT were reported in three MAs (16,24,26) which encompassed 8–14 RCTs (519–867 participants). The pooled results demonstrated that XBJ plus RT could considerably shorten the APTT and PT when compared with RT alone. The quality of evidence for APTT was low to moderate and for PT was low.
Discussion
Main findings
In this overview, XBJ has exhibited potential effectiveness in reducing or improving the relevant outcomes, reflecting its possible clinical effectiveness on sepsis. It may be manifested in the improvement of non-endpoint outcomes: inflammation (WBC, PCT and CRP) and coagulopathy (PLT, shorting APTT and PT); the clinical endpoint: 28-day mortality, and a long-term prognosis indicator: APACHE II scores.
AMSTAR 2 and the GRADE system were used to assess the methodological quality and evidence quality of 12 MAs of XBJ for sepsis. The results for methodological quality using AMSTAR 2 showed that all of the reviews were rated as “critically low”. Consistent drawbacks of methodology included the following: (I) lack of a prior protocol. Advanced registration can help to promote processing transparency and avoid post-decision bias (31). (II) Lack of explanation for the selection of the type of study for inclusion. This may lead to selection bias and lower credibility of the results. (III) Lack of a list of excluded studies. The availability of exclusion lists reduces selectivity bias and ensures research transparency. (IV) Lack of reports on sources of research funding. It is important to avoid other biases as the results of researches that receive corporate funding are more beneficial to the funder (32). Therefore, the absence of any of the above factors will reduce the credibility of the research results. We should avoid the occurrence of these problems in the future.
According to the GRADE system, this overview of 12 reviews and 45 outcomes showed that only 1 (2.2%) outcome was rated as high quality, 10 (22.2%) were rated as moderate quality, 28 (62.2%) were rated as low quality, and 6 (13.3%) were rated as very low quality. The main factors for downgrading evidence quality are as follows: (I) risk of bias, almost all the outcomes related to RCTs designed with a large bias in random sequence generation, allocation concealment, blinding, or incomplete outcome data. Due to the particularity of XBJ (a brown color liquid), blinding participants is difficult, which would require covering the bottles and transmission pipes during transfusion. (II) Imprecision, 17 (37.8%) outcomes were downgraded for small sample size and wide confidence interval, so large sample size RCTs are needed. (III) Inconsistency, 24 (53.3%) outcomes were downgraded due to inconsistency. Therefore, it is essential to perform the subgroup analysis to consider possible factors such as XBJ dose, diagnostic criteria, etc., in order to reduce inconsistencies. Despite the potential efficacy of XBJ, the strength of evidence for all outcomes is still unsatisfactory.
Interpretation to efficacy of XBJ
In this overview, the potential effectiveness of XBJ on sepsis may be manifested in the improvement of non-endpoint outcomes: inflammation (WBC, PCT and CRP) and coagulopathy (PLT, shorting APTT and PT); the clinical endpoint: 28-day mortality, and a long-term prognosis indicator: APACHE II scores.
Although the quality of evidence for most of the results was low, the efficacy of XBJ in treating sepsis was worthy of recognition. Twenty-eight-day mortality is the most appropriate endpoint in sepsis (33). In our results, only one review (25) showed that XBJ could not effectively reduce 28-day mortality at a dose of 200 mL/d. However, due to the low quality of evidence, the reliability of this result was reduced. Meanwhile, such a result suggested that it may be necessary to explore the effects of different doses of XBJ on the prognosis of patients with sepsis in future studies.
APACHE II scores were used to predict hospital mortality in septic patients (34), which consist of three parts: 12 acute physiological variables, age and chronic health status (35). Our results revealed that RT combined with XBJ could reduce the APACHE II scores and improve the prognosis of patients. But the credibility of the results is undermined by the fact that only one of the outcomes is high grade in quality and the rest are all very low to low grade. It is suggested that more rigorous experimental design is needed to evaluate this index in the future.
WBC, PCT and CRP are all indicators of inflammation, PCT can reflect the active degree of systemic inflammation, and WBC and CRP are commonly used clinical indicators of inflammatory response. Of the 15 inflammatory outcomes, the positive outcomes were rated low, and the negative outcomes were rated very low. This suggested that positive outcomes may be more reliable, meaning that RT plus XBJ is more effective at reducing inflammation than RT alone. In addition, one review (10) also found that the dose of XBJ had an impact on the above-mentioned WBC, suggesting that subgroup analysis of XBJ dose is particularly necessary. Moreover, many reviews have indicated that XBJ could inhibit the release of pro-inflammatory cytokines such as TNF-α (23,30) and IL-6 (36) in patients with sepsis. There are some studies that have shown that XBJ could also promote the release of anti-inflammatory cytokine IL-10 in the early stage of sepsis (37,38). Further studies suggested that XBJ might play its anti-inflammatory role by down-regulating the expression of NF-κB, MAPK, and PI3K/Akt signaling pathway (39,40). More precise and comprehensive researches into the mechanism are needed.
PLT, APTT and PT are all effective indicators to reflect the coagulation function of the body, and all have strong sensitivity (41). Not only did this overview demonstrated that XBJ could improve coagulation function in patients with sepsis, but other MAs (42,43) had demonstrated this as well. XBJ reduced the release of tissue factor (44,45), increases the levels of plasma activated protein C (46) and inhibits the expression of plasminogen activator inhibitor-1 (47), thereby improving the coagulation dysfunction (12).
Inflammation (48), immunosuppression (49) and coagulation dysfunction (50) are key features in the pathogenesis of sepsis. In addition to the improvement of inflammatory response and coagulation dysfunction mentioned above, XBJ has also been reported to improve the immune function (28,51,52) of patients with sepsis. Therefore, XBJ has the effect of a multi-target treatment and a comprehensive regulation on sepsis. Over the last three decades, most of the therapeutics strategies successful in experimental sepsis failed in the clinical trials (53,54) and sepsis also remains a scientific and clinical challenge. The multi-target therapeutic advantage of XBJ is well-suited to address the critical points of the current sepsis clinical trial failure. Despite the low methodological quality and low evidence quality, it is still a good choice in the situation in which no treatment approved by the FDA is available for sepsis treatment. Moreover, the protection mechanism of XBJ is being further investigated (12,39).
In addition, it is worth nothing that XBJ is not only effective in treating sepsis, but also widely used in severe pneumonia (11), severe heat stroke (55), acute organophosphorus pesticide poisoning (56), rheumatoid arthritis (57) and other diseases. In particular, XBJ has been widely used in the treatment of COVID-19, which is the most pertinent application in the world at this moment, and its effect is remarkable (58-60). Researches on the application of XBJ to different diseases are ongoing.
Strengths and limitations
This overview is the first attempt to assess the methodological quality of SRs and MAs using the AMSTAR 2 tool and GRADE system to evaluate the quality of evidence for the efficacy of XBJ for sepsis. We conducted systematic and comprehensive searches and a reasonable literature screening, which may greatly reduce possible selection bias. Furthermore, this overview included SRs of randomized trials using strict inclusion standards, and excluded reviews with non-RCTs or observational studies in order to reduce the risk of mixed bias.
This overview still has limitations. Firstly, the evaluation process of AMSTAR 2 and GRADE is inevitably subjective and may result in bias. Secondly, the methodological quality and evidence quality of the included MAs were generally low; thus, results should be interpreted with caution. Third, we did not conduct the subgroup analysis and comparison of XBJ dose, so the effect of dose on XBJ efficacy needs further study.
Suggestions for future research
Since the methodological quality and evidence quality of MAs were generally low, the credibility of the results has been reduced, indicating that the efficacy of XBJ on sepsis is limited. We recommend that rigorous RCTs be designed, with attention to specific blinding and allocation concealment. For SRs and MAs, we suggest that subgroup analysis should be performed strictly according to consistent diagnosis and intervention, consistent treatment dose, and outcome measurement so as to reduce bias. In addition, safety evaluation of XBJ is rarely seen in RCTs, and only 2 (14,15) of the RCTs included in the 12 MRs reported adverse reactions such as skin itching and rash after the use of XBJ. The RCTs should give more attention to reporting safety aspects.
Conclusions
In this paper, the inclusion results of MAs were extracted and analyzed systematically, suggesting that XBJ is clinically effective in the treatment of sepsis, especially in reducing inflammation, reducing mortality, improving coagulation dysfunction and prognosis. But this conclusion should be interpreted prudently, given the generally low methodological quality and low quality of evidence of the included MAs. In the future, rigorous MAs are needed following methodological requirements to provide robust evidence for definitive conclusions.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (NSFC) (grant numbers 81774429, 81973952 and 81973951), Natural Science Foundation of Shanghai (grant number 19ZR1451500), Scientific Research Project of Shanghai Municipal Health Commission (grant number 20194Y0164), Shanghai Sailing Program (grant number 20YF1445300), National Key R&D Program of China (grant number 2018YFC1704600).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/lcm-21-13
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/lcm-21-13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020;395:200-11. [Crossref] [PubMed]
- Markwart R, Saito H, Harder T, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med 2020;46:1536-51. [Crossref] [PubMed]
- Prescott HC, Angus DC. Postsepsis Morbidity. JAMA 2018;319:91. [Crossref] [PubMed]
- Prescott HC, Angus DC. Enhancing Recovery From Sepsis: A Review. JAMA 2018;319:62-75. [Crossref] [PubMed]
- Reinhart K, Daniels R, Kissoon N, et al. Recognizing Sepsis as a Global Health Priority - A WHO Resolution. N Engl J Med 2017;377:414-7. [Crossref] [PubMed]
- Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet 2018;392:75-87. [Crossref] [PubMed]
- Polat G, Ugan RA, Cadirci E, et al. Sepsis and Septic Shock: Current Treatment Strategies and New Approaches. Eurasian J Med 2017;49:53-8. [Crossref] [PubMed]
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96. [Crossref] [PubMed]
- Li C, Wang P, Zhang L, et al. Efficacy and safety of Xuebijing injection (a Chinese patent) for sepsis: A meta-analysis of randomized controlled trials. J Ethnopharmacol 2018;224:512-21.
- Song Y, Yao C, Yao Y, et al. XueBiJing Injection Versus Placebo for Critically Ill Patients With Severe Community-Acquired Pneumonia: A Randomized Controlled Trial. Crit Care Med 2019;47:e735-43. [Crossref] [PubMed]
- Li C, Wang P, Li M, et al. The current evidence for the treatment of sepsis with Xuebijing injection: Bioactive constituents, findings of clinical studies and potential mechanisms. J Ethnopharmacol 2021;265:113301 [Crossref] [PubMed]
- Zhang H, Wei L, Zhao G, et al. Protective effect of Xuebijing injection on myocardial injury in patients with sepsis: a randomized clinical trial. J Tradit Chin Med 2016;36:706-10. [Crossref] [PubMed]
- Lin XJ, Tang JW, Zhong LH, et al. Effects of Xuebijing injection on blood coagulation function and prognosis of sepsis patients. China Pharmcy 2016;27:653-4.
- Li QB, Zeng QC, Tong L, et al. Curative effect and possible mechanisms of xuebijing injection in the elderly with sepsis. Journal of Medical Research 2009;38:50-3.
- Hou SY, Feng XH, Lin CL, et al. Efficacy of Xuebijing for coagulopathy in patients with sepsis. Saudi Med J 2015;36:164-9. [Crossref] [PubMed]
- Shi H, Hong Y, Qian J, et al. Xuebijing in the treatment of patients with sepsis. Am J Emerg Med 2017;35:285-91. [Crossref] [PubMed]
- Wu Y, Zhang J, Qi L. Clinical efficacy and safety of Xuebijing injection on sepsis: a Meta-analysis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020;32:691-5. [PubMed]
- Pollock M, Fernandes RM, Becker LA, et al. Chapter V: overviews of reviews. In: Higgins JPT, Thomas J, Chandler J, et al. editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane, 2020.
- International Platform of Registered Systematic Review and Meta-analysis Protocols: Effectiveness of Xuebijing injection for sepsis: overview of systematic reviews and meta-analyses. Available online: https://inplasy.com/inplasy-2020-12-0126/. Accessed 25 Jun 2021.
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Sun CL, Zhuang Y, Wang X. Meta-analysis of Xuebijing injection for the treatment of sepsis. J Emerg Tradit Chin Med 2012;21:411-3.
- Li N, Jiang LW, Yu L, et al. Systematic review of Xuebijing injection for the treatment of sepsis. Chin J Mod Drug Appl 2013;7:8-11.
- Xu YQ, Geng Y, Tong HS, et al. Xubijing injection improving survival rate of sepsis patients: a meta-analysis. Clinical Journal of Traditional Chinese Medicine 2014;26:456-60.
- Sun HS, Lv JJ, Wei J. Effect of Xuebijing Injection on coagulation function in patients with sepsis: a systematic review and Meta-analysis. Clinical Medication Journal 2015;13:41-6.
- Li GZ, Xiao Y, Zhu JW, et al. Systematic review of Xuebijing injection for the treatment of septic shock. J Emerg Tradit Chin Med 2016;25:834-8.
- Xu YQ. The systemic review of xuebijing for treating immune dysfunction in sepsis. China: Guangzhou University of Chinese Medicine, 2016.
- Zhou XS, Tang GH, Li J, et al. Xuebiing injection reducing mortality of septic shock patients: a systematic review and meta-analysis. Chin Arch Tradit Chin Med 2016;34:2161-4.
- Zhang JW, He YL, Shi Y, et al. Meta-analysis of the efficacy and safety of Xuebijing injection in the treatment of vascular endothelial injury in sepsis. Journal of Hainan Medical University 2021;27:910-7.
- Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev 2012;1:7. [Crossref] [PubMed]
- Schmieder RE, Neuzil P. Scientific Data and Transparency of Conflict of Interest Are Important, Not Biased Editorial Without Facts. JACC Cardiovasc Interv 2016;9:2263. [Crossref] [PubMed]
- Vincent JL. Endpoints in sepsis trials: more than just 28-day mortality? Crit Care Med 2004;32:S209-13. [Crossref] [PubMed]
- Sadaka F. Predicting Mortality of Patients With Sepsis: A Comparison of APACHE II and APACHE III Scoring Systems. J Clin Med Res 2017;9:907-10. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Xiao SH, Luo L, Liu XH, et al. Curative efficacy and safety of traditional Chinese medicine xuebijing injections combined with ulinastatin for treating sepsis in the Chinese population: A meta-analysis. Medicine (Baltimore) 2018;97:e10971 [Crossref] [PubMed]
- Li A, Li J, Bao Y, et al. Xuebijing injection alleviates cytokine-induced inflammatory liver injury in CLP-induced septic rats through induction of suppressor of cytokine signaling 1. Exp Ther Med 2016;12:1531-6. [Crossref] [PubMed]
- Liu YC, Yao FH, Chai YF, et al. Xuebijing Injection Promotes M2 Polarization of Macrophages and Improves Survival Rate in Septic Mice. Evid Based Complement Alternat Med 2015;2015:352642 [Crossref] [PubMed]
- Li T, Qian Y, Miao Z, et al. Xuebijing Injection Alleviates Pam3CSK4-Induced Inflammatory Response and Protects Mice From Sepsis Caused by Methicillin-Resistant Staphylococcus aureus. Front Pharmacol 2020;11:104. [Crossref] [PubMed]
- Jiang M, Zhou M, Han Y, et al. Identification of NF-κB Inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J Ethnopharmacol 2013;147:426-33. [Crossref] [PubMed]
- Ding H, Cao XY, Ma XG, et al. Endothelial cell injury with inflammatory cytokine and coagulation in patients with sepsis. World J Emerg Med 2013;4:285-9. [Crossref] [PubMed]
- Peng DM. Clinical efficacy of Xuebijing combined with ulinastatin in treatment of burn sepsis: a systematic review. Tianjin Journal of Traditional Chinese Medicine 2019;36:258-63.
- Xu YQ. Meta-analysis of randomized controlled trials of Chinese patent drug Xuebijing in the treatment of sepsis patients. China: Southern Medical University, 2014.
- Gui YG, Yao YM, Chai YF. The effects of Xuebijing injection on expression of tissue factor in rat monocytes stimulated with lipopolysaccharide in vitro. Chin J Exp Surg 2009;26:289-91.
- Gui YG, Yao YM, Chai YF. A comparison of interference effect between Xuebijing injection and activated protein C on lipopolysaccharide-induced tissue factor and protease activated receptor-1 expressions on monocytes in rats. Chin J TCM WM Crit Care 2009;16:326-9.
- Li YP, Zheng GJ, Wu ZX, et al. Effects of Xuebijing injection on activated protein C and coagulation parameters in septic rats. Chin J TCM WM Crit Care 2008;15:361-4.
- Zhang ZL, Shen QQ, Zhu J, et al. The effect of Xuebijing on expression of PAI-1 in lipopolysaccharide-induced acute lung injury in rats. Chin J Clin Healthc 2011;14:163-6+225.
- Brooks D, Barr LC, Wiscombe S, et al. Human lipopolysaccharide models provide mechanistic and therapeutic insights into systemic and pulmonary inflammation. Eur Respir J 2020;56:1901298 [Crossref] [PubMed]
- Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol 2018;14:121-37. [Crossref] [PubMed]
- Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, et al. The pathogenesis of sepsis. Annu Rev Pathol 2011;6:19-48. [Crossref] [PubMed]
- Chen X, Feng Y, Shen X, et al. Anti-sepsis protection of Xuebijing injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. J Ethnopharmacol 2018;211:358-65. [Crossref] [PubMed]
- Ma ST, Yu H, Zhang XL, et al. Potency Material Bases of Xuebijing Formula and Its Multi-target Effects on Sepsis. Zhongguo Zhong Xi Yi Jie He Za Zhi 2015;35:1351-5. [PubMed]
- Kumar V. Sepsis roadmap: What we know, what we learned, and where we are going. Clin Immunol 2020;210:108264 [Crossref] [PubMed]
- Artenstein AW, Higgins TL, Opal SM. Sepsis and scientific revolutions. Crit Care Med 2013;41:2770-2. [Crossref] [PubMed]
- Jin H, Chen Y, Ding C, et al. Microcirculatory Disorders and Protective Role of Xuebijing in Severe Heat Stroke. Sci Rep 2018;8:4553. [Crossref] [PubMed]
- Huang P, Li B, Feng S, et al. Xuebijing injection for acute organophosphorus pesticide poisoning: a systematic review and meta-analysis. Ann Transl Med 2019;7:112. [Crossref] [PubMed]
- Li S, Wang H, Sun Q, et al. Therapeutic Effect of Xuebijing, a Traditional Chinese Medicine Injection, on Rheumatoid Arthritis. Evid Based Complement Alternat Med 2020;2020:2710782 [Crossref] [PubMed]
- Guo H, Zheng J, Huang G, et al. Xuebijing injection in the treatment of COVID-19: a retrospective case-control study. Ann Palliat Med 2020;9:3235-48. [Crossref] [PubMed]
- Ma Q, Qiu M, Zhou H, et al. The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical Corona Virus Disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacol Res 2020;160:105073 [Crossref] [PubMed]
- Wen L, Zhou Z, Jiang D, et al. Effect of Xuebijing injection on inflammatory markers and disease outcome of coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020;32:426-9. [PubMed]
Cite this article as: Shi YL, Chen CT, Yang YQ, Wang Y, Chen YJ, Xu YD. Effectiveness of Xuebijing injection for sepsis: an overview of systematic reviews and meta-analyses. Longhua Chin Med 2021;4:24.