
A narrative review of curcuminoids from various Curcuma species in Indonesia as potential antidiabetic agents
Introduction
Diabetes mellitus (DM) is considered a disease having the characteristic of inadequate control of blood glucose levels in the body, which is usually associated with high glucose levels or hyperglycemia. Two main conditions that may cause DM are insufficient insulin production and the inability of the body to properly use insulin or termed insulin resistance (1). The DM can be classified as type 1 and type 2 depending on the DM cause. Type 1 DM that is often called insulin-dependent DM is caused by the inability of the body to produce insulin, which is normally secreted from the β cell of pancreas. Meanwhile, type 2 DM is defined as a noninsulin-dependent DM caused by the relative decrement of insulin activity as the result of insulin resistance where the body cells in the muscles, fat, and liver can not respond well to the produced insulin. This symptom can be triggered by an unhealthy dietary intake (2). DM is also often related to major health complications such as pathogenesis diabetic complication, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, macrovascular complications, and miscellaneous complications (3). Due to the several major complications, DM has been reported as one of the fatal diseases and the third disease leading cause of death (4). Specifically in Indonesia, DM has been one of the major health problems since the early 1980s. Indonesia has more than 10 million people who suffer from this disease with a prevalence rate of 6.2%, and also high major death caused by the DM (5). Besides, Indonesia has been reported as one of the top ten global countries with a high number of DM patients in 2013. Even in the future, the same condition has been predicted to keep continue, unless the proper treatment and management of DM cases has been applied (6).
While different types of DM would require different medication treatments, several other factors such as age, gender, and congenital disease are also considered in DM medication (7). Medication for type 1 DM can be done by adding insulin from an external source, known as injectable insulin, which was discovered in early 1920 (8). However, in several cases, the use of insulin can cause several major side effects, such as hypoglycemia, increased body weight, lipodystrophy, antibody-mediated insulin resistance, postural hypotension, edema, and several allergic reactions. Besides, insulin is relatively expensive due to its low bioavailability and complex production (9,10). Consequently, all of these factors lead to the limited use of insulin as it can not be used for all diabetic patients. On the other side, DM type 2 uses different treatments which are motsly focusing on drug medication. The drug used in this treatment should have at least one of these functions, i.e., antihyperglycemic effect, the insulin sensitizer, or inhibition of enzyme corresponding to glucose production. Several commercial drugs have been developed such as metformin, acarbose, voglibose, meglitinide, and rosiglitazone (11). Unfortunately, the use of these drugs often cause some adverse effects. In some cases, the use of metformin can cause gastrointestinal symptoms, nausea, vomiting, and also lactic acidosis symptoms (1). Meanwhile, the use of drug-based α-glucosidase inhibitors can cause few side effects such as diarrhea, flatulence, elevated transaminases, and abdominal pain (11). Considering it possible to give the adverse effects, the use of these commercially available drugs is also limited for certain patients only.
In recent decades, many researchers work to develop herbal medicine as an alternative for the treatment of DM with negligible adverse effects. The use of both traditional Chinese medicine (TCM) and traditional Indian medicine (TIM) has been reported to be effective as antidiabetic agents (12). Many herbal medicines also have other biological active properties such as antioxidant, anti-inflammatory, regulating blood lipid, and regulating gut microbiome which may provide a synergistic effect in DM therapy (13). While over more than 400 plant extracts have been shown to have antidiabetic activity, these bioactive compounds can be generally classified into anthocyanine, tannins, polyphenol, alkaloid, flavonoid, and curcuminoids (14). Among them, curcuminoids have been widely used for DM treatment because of its potential antidiabetic activity and also high availability. Curcuminoids have been also reported to have several other bioactivities such as antioxidant, anti-inflammatory, and immunomodulatory. Generally, curcuminoids are present in the turmeric plants, which belong to the Curcuma species, and its mechanism of antidiabetic activity depending on the functional groups of the curcuminoids (14,15).
Indonesia has wide biodiversity of plant species that can be used as the sources of bioactive compounds (16). Curcuma species is considered one of the natural plants which are often used as herbal medicine in Indonesia. Four types of the Curcuma species in Indonesia including Curcuma longa (turmeric or rimpang kunyit), Curcuma heyneana (temu giring), Curcuma zanthorrhiza (Java turmeric or temulawak), and Curcuma mangga (temu mangga) are the common species widely used in herbal medicine since they contain curcuminoids as one of the active compounds (17-20). The composition and the activity of the curcuminoids have been reported to depend on the location where the plant is harvested (21). Considering the potential activity of curcuminoids as the antidiabetic agent, this review aims to explore the application of various Curcuma species containing curcuminoids in Indonesia for antidiabetic treatment.
The overview of this topic was mostly retrieved from search engines in internet databases such as Scholar Google (December 2020–May 2021). The comparison of antidiabetic potentials was discussed based on four Curcuma species in Indonesia. We present the following article in accordance with the Narrative Review checklist (available at https://dx.doi.org/10.21037/lcm-21-9).
Structure and composition of curcuminoids
Curcuminoids are the family of bioactive compounds in Curcumin species and are widely recognized as natural phenolic compounds. Having the phenol groups in its molecular structure, curcuminoids possess antioxidant activity (22,23). In general, the structure of curcuminoids have three important parts, which are two phenol aromatic groups, α, β-unsaturated β-diketone moiety (keto-enol group), and seven carbon aliphatic group as a linker of two aromatic rings (24), as depicted in Figure 1. Curcuminoids is usually found as a mixture of three main compounds, namely curcumin, demethoxycurcumin, and bisdemethoxycurcumin. The curcumin has both methoxy groups as the R1 and R2 substituents. Meanwhile, the demethoxycurcumin has hydrogen and methoxy groups as the substituents, and the bisdemethoxycurcumin only has hydrogen as its substituents.
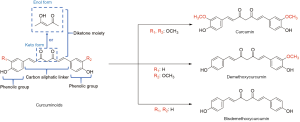
The composition of the three main compounds in the curcuminoids is varied, depending on the type of the Curcuma species. Among these three compounds, the curcumin has been recognized as the most active component for the diabetes treatment (25). Table 1 shows the examples of reported curcuminoids composition in several Curcuma species. While the demethoxycurcumin is the main compound found in the Curcuma mangga (19) and Curcuma heyneana (20), the curcumin is found as the major compound of curcuminoids in Curcuma longa (26,27) and Curcuma zanthorrhiza (28,29). The works also showed that the composition of the same Curcuma species could be slightly different from each other. All these studies showed that two or more compounds exist in the curcuminoids extracted from the Curcuma species.
Table 1
| Species | Relative composition (%) | Ref. | ||
|---|---|---|---|---|
| Curcumin | Demethoxycurcumin | Bisdemethoxycurcumin | ||
|
|
1.52 | 56.89 | 41.60 | ( |
|
|
13.93 | 71.52 | 14.55 | ( |
|
|
75.00 | 20.00 | 5.00 | ( |
|
|
67.31 | 23.81 | 13.88 | ( |
|
|
70.03 | 25.91 | 4.06 | ( |
|
|
73.10 | 26.90 | ND | ( |
| ND, not detected. | ||||
Antioxidant and antidiabetic properties of curcuminoids
The antioxidant capacities and activities of curcumin, demethoxycurcumin, and bisdemethoxycurcumin from the curcumin removed turmeric oleoresin (CRTO) were studied by phosphomolybdenum and linoleic acid peroxidation methods, respectively (30). The results showed that curcumin gave the highest antioxidant capacities, followed by demethoxycurcumin and bisdemethoxycurcumin, which were 3,099, 2,833, and 2,677 µmol/g, respectively. The values were equivalent to 50 ppm of ascorbic acid. Meanwhile, the antioxidant activities were also obtained in the same order of curcumin (81.98%), demethoxycurcumin (81.77%), and bisdemethoxycurcumin (73%). Another report revealed the antioxidant activity of the curcuminoids by using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method (31). Their IC50 values were obtained as 35.1, 53.4, and >200 µM for curcumin, demethoxycurcumin, and bisdemethoxycurcumin, respectively. This result again confirmed that curcumin has better antioxidant activity than other curcuminoids. It was proposed that the difference in their antioxidant activities might be affected by the differences in the aromatic substituents.
The antioxidant activity of a compound is determined by the ability of the compound in transferring a hydrogen atom to free-radical species (24,30). The compound would scavenge itself to convert the free-radical species into unharmful species. In the case of curcuminoids, the presence of the phenolic group takes a pivotal part in the scavenging process of reactive oxygen species (ROS) (24,31). However, the existence of the phenolic group may not be the only reason why curcumin achieves higher activity than the others. Curcumin has the methoxy group in both phenol aromatic rings that can give a positive impact on the hydrogen atom transfer process. When the methoxy group is located near the phenol hydroxyl group, they would form an intramolecular hydrogen bonding promoting the release of the hydrogen atom from the methoxy group easier (31). Hence, this also confirms why the antioxidant activity of demethoxycurcumin was closer to curcumin than bisdemethoxycurcumin.
The interactions between curcuminoids and biomolecules have been also studied. Curcuminoids can interact with various biomolecules through either covalent and non-covalent interactions (24). The covalent interactions generally occur when the keto-enol group of curcuminoids interacts with protein thiols or metals. Meanwhile, the interactions such as hydrophobic interaction, π-π interaction, and hydrogen bonding that are known as the non-covalent interaction, can occur on the three functional groups of the curcuminoids. Besides, the computational study showed that curcumin would bind with the protein molecules of enzymes through hydrophobic interaction. The functional group of curcuminoids that responsible for the hydrophobic interaction is the carbon linker group. Even though the carbon linker group does not have an important role in the antioxidant activity of curcuminoids, this group would play a central role in the antidiabetic activity.
Correlating to the antidiabetic properties, curcuminoids would act as the α-amylase and α-glucosidase inhibitors for controlling the glucose level in the blood (25,32,33). The α-amylase enzyme corresponds to the digestion of starch and conversion of glycogen to glucose in the metabolite system, while the α-glucosidase enzyme is responsible to digest carbohydrates to glucose. Therefore, the inhibition of these enzymes would reduce the glucose level in the blood system. As it was revealed that antioxidants would enhance the metabolism of the glucose body system (34,35), curcuminoids having both antioxidant activity and antidiabetic functions would be very useful for DM treatment.
As the clinical study showed that the reactive oxygen species (ROS) is increased under diabetic conditions, the DM has been highly related to the oxidative stress, which is generated from oxidative properties of the ROS toward the cellular system in the body (34,35). The elevated ROS and the reduced body antioxidant have been reported as the initial causes of hyperglycemia and insulin resistance since such conditions would lead to reduced insulin secretion and insulin action. The conditions can also trigger more generated oxidative stress, and thus causing the condition of DM patients to be worse due to DM complications. Therefore, the presence of antioxidant agents such as curcuminoids would help to prevent further oxidative stress that leads to deleterious effects in DM patients.
The curcuminoids is supposed to act as an antidiabetic agent by working in the pancreatic β cell via triggering the release of insulin and decrease its apoptosis (36). Therefore, insulin production will be greatly increased, resulting in a better-controlled blood glucose level in diabetic patients. Meanwhile, curcuminoids can also inhibit the gluconeogenesis process at the liver cells, and thus, the production of glucose can be suppressed. In addition, curcuminoids also decreases the produced glucose through glycolysis and glycogenesis processes. Glycolysis is a metabolic pathway that converts glucose into small molecules such as pyruvate, CH3COCOO−, and hydrogen ion (H+), while the glycogenesis process is the process of producing and storing glycogen which requires glucose molecules. The role of curcuminoid is supposed to increase the glycolysis and glycogenesis processes that can reduce the blood glucose level.
Various Curcuma species in Indonesia and their potential antidiabetic activity
It has been reported that the Curcuma genus has over a hundred species in the world. Based on the data from the Observatory of Economic Complexity (OEC, 2019), Indonesia ranked number four in the world as the top Curcuma (turmeric) exporter. Therefore, in this review, particular attention is made to the four species of the Curcuma genus in Indonesia that have been applied extensively for herbal medicines i.e., Curcuma longa (syn. Curcuma domestica), Curcuma heyneana, Curcuma zanthorrhiza, and Curcuma mangga (17). Moreover, these Curcuma species have been reported to contain curcuminoids as one of the bioactive components (37). The antidiabetic activity of these extracts has been also investigated for herbal medicines application by either in-vivo or in-vitro methods.
Curcuma longa (syn. Curcuma domestica)
The antidiabetic activity of Curcuma longa Linn. (syn. Curcuma domestica Val.) plant species from Indonesia were examined (36). The rhizome of Curcuma domestica Val. was extracted by the maceration method using ethanol. The antidiabetic activity of the ethanolic extract was tested by the in-vivo method using alloxan and glucose-induced diabetic zebrafish. The alloxan was used to cause the pancreatic β cell damage, while glucose was employed to trigger insulin resistance and reach the hyperglycemic condition faster. In this test, the ethanolic extracts at different concentrations of 15.625, 31.25, 62.50, and 125 µg/mL were confirmed to give the glucose-reducing activity. The highest glucose reducing activity was found in the extract concentration of 62.5 and 125 µg/mL, which gave a similar value of 61.43 and 63.14 µg/dL, respectively. Hence, it could be suggested that the optimum dose of this extract was 62.5 µg/mL. It was suggested that the antidiabetic activity would come from the curcumin content in the ethanolic extract of Curcuma domestica Val. rhizome, even though the extract compositions were not identified.
Another research work was reported using the same Curcuma domestica Val. Rhizome (38). The antidiabetic activity was investigated by using the in-vivo method in streptozotocin-induced diabetic rats. The study used the herbal medicine drink from Java called Jamu Gendong Kunyit Asam in Indonesia. This herbal medicine was produced from the combination of Curcuma domestica Val. rhizome and Tamarindus indica L. Combination of these plants resulted in the combined extract contain curcuminoids from Curcuma domestica Val. rhizome and other compounds as saponin, phenolic, and ascorbic acid from Tamarindus indica L. Other active compounds were also identified such as alkaloid, flavonoid, and triterpenoid/steroid. The antidiabetic activity was evaluated from the two-parameter of blood glucose level and the detriment of the islets of Langerhans pancreas. The crude extract of these plant components in the water at two different doses of 1.9 mL/200 g and 3.8 mL/200 g body weight (BW) gave a reduced glucose level of more than 100%. In addition, these two doses show significant activity to prevent the detriment of the islets of Langerhans pancreas caused by the addition of streptozotocin up to 66%. These results were superior when compared to the use of glibenclamide drug which only gave less than 50% reducing blood glucose level and 17% prevention of the islets of Langerhans pancreas detriment. This herbal medicine was potential to be applied for the treatment of DM with the optimum dose of 1.9 mL/200 g BW. Besides the synergistic effect of each component in this herbal medicine, the curcuminoids was supposed to give activity in reducing blood glucose level by its antioxidant properties. In the case of the pancreatic β cell, curcuminoids would repair the detriment cell. This means that the curcuminoids also can provide a triggering effect in the production of insulin from the pancreatic β cell, resulted in the decrease of the blood glucose levels.
A recent research work revealed that the methanolic plant extract of the Curcuma domestica Val. rhizome obtained from Bogor, West Java contained curcumin (16.92%), bisdemethoxycurcumin (5.27%), curcumol (15.51%), and other components considered as terpenoids (39). Although the curcuminoids were not suggested as the main component of this extract, the methanolic plant extract showed potential results both in the antioxidant and antidiabetic activities. The plant extract exhibited a comparable antioxidant activity examined through DPPH method (IC50 =8.33 µg/mL) against the curcumin standard (IC50 =7.85 µg/mL) (40), and superior activity against bisdemethoxycurcumin (IC50 =64.94 µg/mL). On the other side, the antidiabetic activity of this plant extract was tested by the in-vitro method using standard enzymes. It was found that the antidiabetic activity of the plant extract came from the inhibition activity of enzymes such as, α-glucosidase (IC50 =17.18 µg/mL), β-glucosidase (IC50 =2.72 µg/mL), and α-amylase (IC50 =13.25 µg/mL) with inhibition value of 64.63%, 70.84%, and 75.69%, respectively. These inhibitory activity values were slightly weaker than both curcumin and bisdemethoxycurcumin standard, but they were still higher as compared to the standard of acarbose drug inhibitory activity toward glucosidase (IC50 =18.12 µg/mL) and amylase (IC50 =296.3 µg/mL). This study gave clear information that the extract of Curcuma domestica Val. exhibited antidiabetic activity towards the main inhibition of β-glucosidase, and α-amylase enzymes, and also showed minor inhibitory activity towards α-glucosidase enzyme.
Curcuma heyneana
Another species from the Curcuma genus is Curcuma heyneana, which also belongs to the medical plants. The antidiabetic activity of this plant rhizome extract was examined through the in-vivo method using streptozotocin-induced diabetic rats (41). The Curcuma heyneana rhizome was obtained from Batu City, East Java, and extracted in ethanol solvent. It was demonstrated that three different doses of this plant extract at 36, 72, and 108 mg/kg BW gave the different antidiabetic activities. The parameter used for measuring the antidiabetic activity is the amount of superoxide dismutase (SOD) enzyme, which plays a role to inhibit the potentially harmful superoxide species in the cell from damaging the tissue. The SOD enzyme would decrease if the hyperglycemia condition is reached since it induces the formation of the superoxide radical. This means that the SOD acts as the antioxidant in the rat’s body. The rats were injected with streptozotocin to damage the pancreatic β cell, which induced diabetic conditions in the rats. The SOD activity was then measured and compared to those obtained on the rats having the plant extract treatment. The results showed that the optimum dose of Curcuma heyneana extract of 72 mg/kg BW provided an increased SOD activity to 54.167 U/mL, close to the control of normal rats (59.332 U/mL). It was also found that the ethanolic extract of this plant at three different doses could inhibit the damage of pancreatic β cells as well as repair the damaged cell effectively. From the inhibition mechanism of the Curcuma heyneana ethanolic extract, it was suggested that the presence of both hydroxyl groups in both curcumin or flavonoid compounds would act as the hydrogen donating atom to the free radicals. Besides, the resonance from the aromatic ring (conjugated double bond) can make the radicals becoming less reactive. This condition then leads to the prevention of the damaged pancreatic β cell which is highly related to the DM.
Another work investigating the potential of Curcuma heyneana species as the antidiabetic agent was reported, in which the plant rhizome was obtained from North Sumatra (42). The research was conducted by the in-vivo method using maltose-loading diabetic mice. It was found that the maltose-induced diabetic mice reached blood glucose levels of about 228.33 mg/dL after 120 minutes of induction. The treatment of mice using this plant extract at three different concentrations of 50, 100, and 200 mg/kg BW resulted in a significant decrement in blood glucose levels of 102.33, 109.33, and 100.6 mg/dL, respectively. This result showed that the optimum dose was 100 mg/kg BW for the Curcuma heyneana extract. The result was comparable to the activity of the acarbose standard for reducing the blood glucose level under the same condition, which gave the decrement of about 103.16 mg/dL. However, the measurement of blood glucose levels in this study still needs to be optimized. Considering the curcumin active compound in this plant extract, the antidiabetic activity can result from several mechanisms. The mechanisms were observed from the activity of curcuminoids in inhibiting the α-glucosidase enzyme and in stimulating insulin secretion and sensitivity. In addition, it was also suggested that curcuminoids could activate signaling receptors and regulate the required enzyme for glycogenesis. Glycogenesis is a process of glycogen synthesis that requires glucose molecules in the process, and thus, the increase in glycogenesis is related to the reduced blood glucose level.
Curcuma zanthorrhiza
Curcuma zanthorrhiza is another species from the Curcuma genus known as Java turmeric or temulawak and has been used as a traditional herbal medicine for medicinal purposes. The Curcuma zanthorrhiza was reported to contain several active compounds, such as curcuminoids, xanthorrhizol, champhor, zerumbone, geranyl acetate curcumene, and zingiberene (43). It was also found that Curcuma zanthorrhiza extract contains 16.64% xanthorrizhol as its bioactive compound. The potential antidiabetic activity of the Curcuma zanthorrhiza obtained from Jakarta was examined by the in-vivo method using high fat diet-induced obese mice. The plant rhizome was extracted in ethanol solvent. Both the crude Curcuma zanthorrhiza extract and purified xanthorrhizol from the plant extracts were investigated to study their antidiabetic activities. The purified xanthorrhizol at 10 and 25 mg/kg/day exhibited a decrease in the fasting blood glucose level up to 21.8 and 33%, respectively. Meanwhile, the Curcuma zanthorrhiza extract at 50 or 100 mg/kg BW/day gave the value of 28.5% and 31.2%, respectively. From the results, both the Curcuma zanthorrhiza extract and purified xanthorrhizol from the extract showed their higher activity as the antidiabetic agent when compared to the standard of metformin at a dose of 100 mg/kg BW/day. This finding suggested that the antidiabetic activity of Curcuma zanthorrhiza extract came not only from the xhantorrhizol but also from other bioactive compounds such as curcuminoids. The mechanism of its antidiabetic activity was found through attenuating the high fat diet-induced hyperglycemic, insulin resistance, and chronic low-grade systemic inflammatory condition in obese mice.
Another work using the combination of Curcuma zanthorrhiza rhizome and Morinda citrifolia Linn. (Mengkudu fruits) that were obtained from Batu City, East Java was performed by using water as the extracting solvent (44). Through the phytochemical analysis, it was revealed that the Curcuma zanthorrhiza rhizome contained phenolics, alkaloids, flavonoids, and tannins. The curcumin content as one of the phenolic compounds was found to be 553.64 µg/mL in a 345 mL of crude extract obtained from 1,080 g of Curcuma zanthorrhiza rhizome. Meanwhile, the mengkudu fruits were reported to have scopoletin as the active main compound, which is considered to have antidiabetic activity. The antidiabetic activity of this extract was tested by the in-vivo method on the streptozotocin-induced diabetic rats. The combination of Mengkudu (3.6 mL/kg BW) and Curcuma zanthorrhiza (10 mL/kg BW) extracts was found to decrease the blood glucose level more effectively in the first 11 days as compared to the standard of glibenclamide drug (4.5 mg/kg BW). Unfortunately, at 18 days or more, the combination extract showed a slight decrease in capability to decrease the blood glucose, confirming its lower stable properties as compared to the glibenclamide that showed superior ability in decreasing blood glucose.
Curcuma zanthorrhiza obtained from Pontianak, West Kalimantan was analyzed and shown to contain some active compounds, such as phenolic compounds, triterpenoid, flavonoid, tannins, and glycoside (45). The curcumin compound was identified as the phenolic compound in the phytochemical test. The antidiabetic activity of Curcuma zanthorrhiza extracted in ethanol was investigated by the in-vivo method using alloxan (155 mg/kg BW)-induced diabetic rats, which increased the blood glucose level by more than 200 mg/dL. The plant extract was able to significantly decrease the blood glucose level after 7 and 14 days with a decrease of about 133.92 and 142.22 mg/dL, respectively. In addition, the optimum dose of this plant extract to decrease the blood glucose level was 17.5 mg/kg BW, in which the activity was similar to the metformin (63 mg/kg BW). The result suggested that the antidiabetic activity was originated from several active compounds contained in this extract. For example, curcumin played a role in preventing gluconeogenesis in the liver, while flavonoid would act through the dilatation of ROS, and the triterpenoid would play a role in the reduction of insulin resistance by increasing glucose absorption in muscle and fat.
Curcuma zanthorrhiza rhizome obtained from Bandung, West Java was extracted in ethanol and the antidiabetic activity was investigated by the in-vivo method using alloxan (50 mg/kg BW)- induced diabetic rats (46). The antidiabetic activity was evaluated by measuring the plasma glucose levels in the rats. The result showed that the plasma glucose levels were about 516.83, 460.50, and 470 mg/dL after 0, 60, and 120 minutes of alloxan induction, respectively. This plant extract (50 mg/kg) could reduce the plasma glucose level optimally to 236 mg/dL after 120 minutes. The reduced value was superior as compared to the two commercial drugs of metformin and glibenclamide at doses of 195 and 0.65 mg/kg, respectively, where the metformin and glibenclamide could only give the reduced plasma gluocose level to 270.33 and 322.67 mg/dL, respectively. It was also revealed that the extract of Curcuma zanthorrhiza could protect the damaged pancreatic β cell from the induced alloxan and more insulin was produced to give the antidiabetic activity.
The antidiabetic property of another Curcuma zanthorrhiza rhizome obtained from Cimeas, Sukabumi, West Java was also studied (47). Due to the low solubility of curcuminoids in water, the extract of Curcuma zanthorrhiza rhizome containing curcuminoids was impregnated on the solid lipid nanoparticles (Cur-SLN). The antidiabetic activity was then evaluated through the in-vivo method using streptozotocin (50 mg/kg BW)-induced diabetic rats. The different doses of Cur-SLN were used orally in the rats’ specimens at 5, 10, and 20 mg/kg BW every single day up to 15 days. The Cur-SLN at different doses was found to reduce the blood glucose level significantly in the first 7 days. The Cur-SLN dose at 10 mg/kg BW was found to give a superior activity in reducing blood glucose level up to around 84.34 mg/dL after 15 days. Even though the value of reduced blood glucose from this extract was still lower than the standard of glibenclamide drug (93 mg/dL) on the same day, the activity was nearly at a comparable level. On the other side, when the curcumin extract in water was induced into a diabetic rat with a dose of 100 mg/kg BW, the blood glucose level showed an increase over 15 days. This result demonstrated that the curcumin extract in water gave no activity in reducing blood glucose level, and the increased blood glucose level might be caused by streptozotocin. It was suggested that the inactivity of curcumin extract in water was caused by its low solubility in water. The low solubility would result in a slight absorption of the curcumin compound into the body, in other words, would decrease the bioavailability in the body. On the other hand, when the extract was in the form of Cur-SLN, the bioavailability was increased as it would be stabilized in the metabolism system. This combination could result in a significant effect in reducing blood glucose levels.
Curcuma zanthorrhiza rhizome obtained from Bukit Tinggi, West Sumatra was tested by the in-vivo method on the alloxan (65 mg/kg)-induced to the diabetic rats (48). The induction of the ethanolic extract of this plant to the diabetic rat with a dose of 400 mg/kg of BW was found to give a significant activity in reducing the blood glucose level gradually for 7, 14, and 21 days. The optimum reduced blood glucose level by this plant extract was up to 102.8 mg/dL after 21 days of induction. Although the activity of this extract was still lower as compared to the activity of glibenclamide (167.6 mg/dL) at 21 days, it showed a comparable level at 14 days (115.2 mg/dL). This result suggested that the glibenclamide drug was considered to be faster in reducing the blood glucose levels. However, the risk of adverse effects such as hypoglycemia condition would possibly higher as compared to the use of Curcuma zanthorrhiza extract in the long term use.
Curcuma mangga
Curcuma mangga is also one of the Curcuma species which has been used for herbal medicines called Jamu in Indonesia. The potential of Curcuma mangga rhizome obtained from Bantul, Yogyakarta as the antioxidant and antidiabetic agents was examined (49). The antioxidant activity of ethanolic crude extract of this Curcuma mangga as well as its fraction in water, hexane, ethyl acetate, and butanol was tested by using nitrogen monoxide (NO) and hydrogen peroxide (H2O2) scavenging methods. Meanwhile, the antidiabetic activity was tested by the in-vitro method using the α-glucosidase enzyme. The result showed that all the extracts exhibited the scavenging activity as examined by the NO method, with the best activity was observed in the water fraction (IC50 =170.33 µg/L). While the antioxidant activity of this extract was still lower as compared to the standard of butylated hydroxytoluene (BHT) (IC50 =69.75 µg/L), it was nearly similar to acarbose (IC50 =166.00 µg/L). The best H2O2 scavenging activity was obtained in ethyl acetate fraction (IC50 =162.78 µg/L), which was superior to the BHT (IC50 =179.86 µg/L) and acarbose (IC50 =4,421.59 µg/L) by using the same method. It was suggested that the antioxidant activity of this plant extract was originated from the curcuminoids. For the α-glucosidase inhibition activity, the ethanolic crude extract of Curcuma mangga (IC50 =469.69 µg/L) showed a better activity if compared to the standard of acarbose (IC50 =862.93 µg/L). This result confirmed that the Curcuma mangga extract has potential antidiabetic property due to the presence of curcuminoids, which mechanism was proposed to involve inhibition of the α-amylase enzyme.
The antidiabetic activity mechanism of the ethanolic extract of Curcuma mangga was further studied by the in-vitro method using a rat cell-like model (3T3-L1 cell lines) in adipocyte tissue (50). The plant extract at the doses of 200 and 50 µg/mL showed a significant increase in glucose uptake up to 24.49 and 14.71 µM in lipid-laden 3T3-L1 cells. The lipid-laden cells act to store the lipid/fat in the metabolism system. The dose of 200 µg/mL showed a superior glucose uptake as compared to the metformin that gave the glucose uptake of 21.93 µg/mL. A higher glucose uptake implied that the change of glucose in the blood to lipid/fat would be faster, preventing the hyperglycemic condition. The increased GLUT4 gene expression is also a method to evaluate the antidiabetic activity. The GLUT4 corresponds to the insulin-regulated glucose transporter that functioned in glucose uptake. From the GLUT4 gene expression, the plant extract at the doses of 200 and 50 µg/mL showed an increase of mRNA expression of GLUT4 by 8.41 and 11.8 fold, respectively, compared to the normal control. The dose of 50 µg/mL showed a superior activity as compared to the standard of metformin. As the PPAR-gamma activates some genes that increase glucose and lipid uptake, the PPAR-gamma gene expression effect is also a way to measure antidiabetic activity. The PPAR-gamma gene expression using both doses of 200 and 50 µg/mL also showed a significant increase up to 16.27 fold, while metformin gave 23.6 fold. The increased glucose uptake in lipid-laden 3T3-L1 cells, GLUT4 gene expression, and PPAR-gamma gen provided a glucose homeostasis state, which balanced insulin and glucagon and resulted in a controlled blood glucose level. Through this known mechanism, it was evidenced that the ethanolic extract of Curcuma mangga has the potential for antidiabetic activity.
Other research work was also reported utilizing the ethanolic extract of Curcuma mangga obtained from Yogyakarta, Indonesia (51). The extract exhibited an antidiabetic activity by inhibiting α-amylase enzyme through an in-vitro study. The α-amylase enzyme corresponded to the digestion of starch and glycogen to glucose in the metabolite system. Therefore, the inhibition of this enzyme can control glucose uptake and prevent hyperglycemic conditions in the blood. Besides, the ethanolic extract of Curcuma mangga also showed antioxidant activity due to its phenolic compound component, including curcuminoids. The antioxidant activity was measured by the DPPH scavenging method, and it was confirmed that this plant extract showed a superior activity with the IC50 value of 230.94 µg/mL as compared to the standard of acarbose (IC50 =1,102.35 µg/mL). Meanwhile, the α-amylase inhibition by this plant extract gave lower activity with the IC50 value of 363.67 µg/mL when compared to the standard of acarbose (IC50 =61.69 µg/mL). The high antioxidant activity of this plant extract would give a complementary effect on the antidiabetic activity, for instance in the prevention of damaging the pancreatic β cell from scavenging the ROS and other free radicals (52). Since the pancreatic β cell corresponded to the production of insulin, the prevention of its damage could result in a controlled glucose level in the blood, especially for those in DM cases.
Perspectives on the application of Curcuma species as the antidiabetic agent
The information about the Curcuma species involving the identified compounds, proposed mechanism, optimum dose, and the decreased blood glucose level (36,38,39,41-51) are summarized and compared to the metformin standard (53) in Table 2. As shown in Table 2, different Curcuma species, locations, and solvents used to extract the bioactive compounds gave different identified compounds. In general, besides the curcuminoids, terpenoid, flavonoid, tannins, and xanthorrizhol were identified on four types of the Curcuma species in Indonesia. While the detailed compositions of the bioactive compounds are not available, curcuminoids is one of the main constituents found in the Curcuma species (54). Therefore, the rationalization on the origin of antidiabetic activity shall be considered from the curcuminoids.
Table 2
| Species (Location Source) | Identified Compound (Solvent) | Mechanism | Optimum Dose (Method) | Decrease in blood glucose level (Treatment time) |
|---|---|---|---|---|
|
|
Curcuminoids (Ethanol) | Decrease blood glucose level in alloxan-induced diabetic zebrafish by triggering the insulin release and decreasing gluconeogenesis | 62.5 µg/mL ( |
61.43 mg/dL (4 days) |
|
|
Curcuminoids, alkaloid, flavonoid, triterpenoid/steroid (Water) | Decrease blood glucose in streptozotocin-induced diabetic rats by synergic with antioxidant activity in repairing pancreatic β cell and triggering insulin production | 9.5 mL/kg BW ( |
287 mg/dL (28 days) |
|
|
Curcumin, bisdemethoxycurcumin, curcumol, and terpenoid (Methanol) | Inhibit α-glucosidase (minor), β-glucosidase and α-amilase (major) | 17.18, 2.72, and 13.25 µg/mL ( |
NA |
|
|
Curcumin, flavonoid (Ethanol) | Increase the SOD activity in streptozotocin induced-diabetes mellitus in rats by inhibiting damaged pancreatic β cell | 72 mg/1 kg BW/day ( |
NA |
|
|
Curcuminoids, flavonoid, and tannins (Ethanol) | Reduce blood glucose level of maltose loaded mice by inhibiting α-glucosidase enzyme, and stimulating insulin secretion and sensitivity | 100 mg/kg BW/day ( |
109.33 mg/dL (120 mins) |
|
|
Curcuminoids and xanthorrizhol (Ethanol) | Antihyperglycemic and anti-inflammatory activity in high-fat diabetic obese mice by attenuating high fat diet-induced hyperglycemic, insulin resistance, and chronic low-grade systemic inflammatory condition | 100 mg/kg BW/day ( |
151.00 mg/dL (112 days) |
|
|
Curcumin and xanthorrhizol (Water) | Hypoglycemic and pancreas protection in streptozotocin-induced diabetic rats (synergic with |
10 mL/kg BW/day ( |
215 mg/dL (11 days) |
|
|
Curcumin (phenolic), flavonoid, tannins, and triterpenoid (Ethanol) | Reduce glucose level in alloxan-induced diabetic rats by the prevention of gluconeogenesis in the liver, reduction of ROS and insulin resistance | 17.5 mg/kg BW/day ( |
142.22 mg/dL (14 days) |
|
|
Curcumin (phenolic), flavonoid, tannins, and triterpenoid (Ethanol) | Reduce plasma glucose level in alloxan-induced diabetic rats by protecting pancreatic β cell damage, and stimulate insulin production | 50 mg/kg BW/day ( |
252.5 mg/dL (120 mins) |
|
|
Curcumin, demethoxycurcumin, and bisdemethoxycurcumin (Water) | Reduce glucose level in streptozotocin-induced diabetic rats (mechanism not explained in detail) | 10 mg/kg BW/day ( |
84.34 mg/dL (15 days) |
|
|
Xanthorrhizol and curcuminoids (Ethanol) | Reducing blood glucose level in alloxan-induced diabetic rats (mechanism not explained in detail) | 400 mg/kg BW/day ( |
102.8 mg/dL (21 days) |
|
|
Curcuminoids (Ethanol) | Reducing blood glucose level by inhibiting α-glucosidase enzymes | 469.69 µg/mL ( |
NA |
|
|
Curcuminoids and tannins (Ethanol) | Control blood glucose level by increasing glucose ingestion as well as reducing insulin resistance by improving GLUT4 mRNA and PPAR-gamma gene expression | 200 and 50 µg/mL ( |
NA |
|
|
Curcuminoids, terpenoid, saponins, tannins, flavonoid alkaloid (Ethanol) | Reducing blood glucose level by inhibiting the α-amylase enzyme | 363.67 µg/mL ( |
NA |
| Metformin standard ( |
Metformin (Water) | Reduce blood glucose level through inhibition of hepatic gluconeogenesis | 250 mg/kg BW/day ( |
56.66 mg/dL (28 days) |
BW, body weight; NA, no available information.
The capability of the curcuminoids in decreasing the blood glucose level has been proposed and also summarized in Table 2. In general, curcuminoids can decrease the blood glucose level from two different pathways as the bioactive effect is related to insulin production and glucose metabolism. Furthermore, the antioxidant effect of curcuminoids can prevent the harmful effect caused by ROS, which causes the generation of oxidative stress. As shown in Table 2, specifically, curcuminoids can reduce the blood glucose level via several mechanisms, which are inhibiting gluconeogenesis (36,45), decreasing the pancreatic β cell apoptosis (38,41), triggering the release of insulin in the pancreatic β cell (38,42,46), inhibiting the α-amylase and α-glucosidase (39,42,49,51), attenuating high fat diet-induced hyperglycemic and chronic low-grade systemic inflammatory condition (43), protection from insulin resistance (43-46,50), reducing the lipid peroxidase activity (44), reducing ROS (45), and increasing the metabolism of glucose body system (50). Unfortunately, to date, there is still no clear pathway on how curcuminoids can work as the antidiabetic agent related to the specific structure of the compounds, namely curcumin, demethoxycurcumin, and bisdemethoxycurcumin.
Since different mechanisms are proposed, the activity of the curcuminoids as antidiabetic agents could be determined from different parameters such as the reduction of the blood glucose level and increase of SOD activity. Table 2 lists the optimum doses of Curcuma species extracts that gave the optimum antidiabetic activity. For the sake of comparison, the following discussion on the optimum dose was only correlated to the decrease in the blood glucose level (in-vivo method). It was obvious that the optimum dose of each Curcuma species to give the optimum decreased blood glucose level was different from each other. Different Curcuma species gave different optimum doses due to the different bioactive compounds as well as the different solvents used to extract the compounds. However, it was also shown that even though the same solvent was used, the same Curcuma species but from different locations did not give the similar optimum dose. As an example, using ethanol as the solvent, the Curcuma zanthorrhiza taken from Jakarta showed the optimum dose of 100 mg/kg BW/day, giving the decrease of blood glucose level up to 151 mg/dL (43). In contrast, a lower optimum dose of 17.5 mg/kg BW/day resulted in a similar level of blood glucose level (142.22 mg/dL) when using the Curcuma zanthorrhiza taken from West Kalimantan (38). Curcuma zanthorriza from the same area of West Java but different cities of Bandung (39) and Sukabumi (40) were also shown to give different optimum doses (50 and 10 mg/kg BW/day) and different decreases in blood glucose level (252.5 and 84.34 mg/kg BW/day), which could be due to the different used solvents (ethanol and water) and also an indication that the location where the Curcuma species was planted could lead to the different antidiabetic activities.
As different conditions were employed in the experiments to determine the antidiabetic activities, a universal index shall be used to compare the antidiabetic activities. An antidiabetic index can be proposed here as the ratio between the decrease of blood glucose level (in mg/dL) and the optimum dose (in mg/kg BW/day) used to achieve such a decrease. The better the antidiabetic activities, the higher the antidiabetic index values. For example, when using metformin with an optimum dose of 250 mg/kg BW/day, the decrease of blood glucose level up to 56.66 mg/dL can be expected (53) and this gives an antidiabetic index of 0.23. Similar calculations were carried out for all the Curcuma species shown in Table 2 and the antidiabetic index values were varied in the range of 0.26–8.13. The wide range showed the variation of the antidiabetic activities of these Curcuma species. However, it was obvious that the Curcuma species gave a similar and even higher ability to decrease the blood glucose level than the metformin. This result indicated that the curcuminoids from Curcuma species extract can potentially be used for DM treatment.
The non-toxicity properties of curcuminoids as antioxidant and antidiabetic agents have been studied (55-58). It was revealed that the curcuminoids from natural sources showed minimum adverse effects, and thus, safe to be used for medicinal application. For instance, the curcumin capsule having 98% purity gave no clinical toxicity effect at the dosage of 50 mg (55). Furthermore, no adverse effect was observed when the curcumin was used for the treatment of arthritis at an even higher dosage of 1,200 mg curcumin/day for 2–6 weeks (56). In addition, it was reported that the usage of curcumin up to 8,000 mg/day showed no indication of treatment-related toxicity (57). In contrast, a commercial drug such as metformin at a lower dosage of 1,000 mg/day caused adverse effects of developed purpuric skin lesions (58).
Besides the capability to reduce the blood glucose level, the non-toxicity and safe use of curcuminoids as well as the possibility to obtain the curcuminoids from Curcuma species would be the important facts to be considered to use the curcuminoid for the DM medication. However, even though all the Curcuma species showed potential antidiabetic activity, the standards in doing the extraction and activity test have not been available yet. Moreover, the stability of the extract also raised questions and should be developed further. For instance, curcumin has been reported to have chemical instability, poor photostability, short half-life, and rapid metabolism (59). Curcumin is photosensitive and pH-sensitive as it can be easily degraded to ferulic acid, feruloyl methane, ferulic aldehyde, vanillic acid, and vanillin due to photodegradation and alkaline degradation.
Owing to the chemical structure of curcuminoids having hydrophobic parts, the curcuminoids show low solubility in water, which means also has low bioavailability. These limitations raised challenges in the formulation of curcuminoids for drug application. Several methods have been reported to overcome this problem, such as modification with phospholipid complexes, hydrophilic carrier, polymer-based nanoparticle, and lipid-based nanoparticles (59), as well as via an impregnation on the solid lipid nanoparticles (47). Some studies have been also explored to find suitable solvents to create water-soluble curcuminoids (60,61).
Conclusion
Curcuminoids is considered a mixture of bioactive compounds that mainly exist as curcumin, demethoxycurcumin, and bisdemethoxycurcumin compounds. Curcuminoids has been confirmed not only to have antioxidant activity but also antidiabetic activity. As the sources of curcuminoids, Curcuma genus has various species spread around the world, including Indonesia. Specifically in Indonesia, Curcuma longa, Curcuma heyneana, Curcuma zanthorrhiza, and Curcuma mangga are known as the main Curcuma species that have extensively been used for the production of herbal medicines. All these Curcuma species show antidiabetic activity either evaluated by the in-vivo or in-vitro methods. The ability of curcuminoids in the Curcuma plant extract to trigger the release of insulin and prevent the damage of pancreatic β cells was tested through the in-vivo methods. Meanwhile, the ability of curcuminoids in the α -glucosidase, β-glucosidase, and α-amylase enzyme inhibition to control the blood glucose level was tested by the in-vitro method. Their antidiabetic activity values have been reported to be comparable and in some cases even higher than the value of commercial drug standards such as metformin, acarbose, and glibenclamide. Hence, curcuminoids could be proposed as a safe compound for reducing blood glucose levels in long-term use. However, the different location sources of Curcuma species in Indonesia also affected the curcuminoids composition and its antidiabetic activity. Moreover, the low stability and the low bioavailability of the curcuminoids in a crude extract of Curcuma species still need to be overcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Lee Suan Chua) for the series “Bioactive compounds from natural products with antidiabetic potentials” published in Longhua Chinese Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/lcm-21-9
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/lcm-21-9). The series “Bioactive compounds from natural products with antidiabetic potentials” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval was not required for this study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Les F, Cásedas G, Gómez C, et al. The role of anthocyanins as antidiabetic agents: from molecular mechanisms to in vivo and human studies. J Physiol Biochem 2021;77:109-31. [Crossref] [PubMed]
- Gunathilaka TL, Samarakoon K, Ranasinghe P, et al. Antidiabetic Potential of Marine Brown Algae-a Mini Review. J Diabetes Res 2020;2020:1230218 [Crossref] [PubMed]
- Papatheodorou K, Banach M, Edmonds M, et al. Complications of Diabetes. J Diabetes Res 2015;2015:189525 [Crossref] [PubMed]
- Bhattacharjee R, Mitra A, Dey B, et al. Exploration of anti-diabetic potentials amongst marine species-a mini review. Indo Glob J Pharm Sci 2014;4:65-73. [Crossref]
- Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137-49. [Crossref] [PubMed]
- Ligita T, Wicking K, Francis K, et al. How people living with diabetes in Indonesia learn about their disease: A grounded theory study. PLoS One 2019;14:e0212019 [Crossref] [PubMed]
- Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev 2016;37:278-316. [Crossref] [PubMed]
- Vaz JA, Patnaik A. Diabetes mellitus: Exploring the challenges in the drug development process. Perspect Clin Res 2012;3:109-12. [Crossref] [PubMed]
- Sahasrabudhe RA, Limaye TY, Gokhale VS. Insulin Injection Site Adverse Effect in a Type 1 Diabetes Patient: An Unusual Presentation. J Clin Diagn Res 2017;11:OD10-1. [Crossref] [PubMed]
- Kashi Z, Akha O, Borzouei S, et al. Insulin therapy: side effects and their management. Clin Exc 2013;1:1-16.
- Pontarolo R, Sanches ACC, Wiens A, et al. Pharmacological treatments for type 2 diabetes, treatment of type 2 diabetes. Colleen Croniger, IntechOpen (April 1st 2015);doi:
10.5772/59204 .10.5772/59204 - Wang Z, Wang J, Chan P. Treating type 2 diabetes mellitus with traditional chinese and Indian medicinal herbs. Evid Based Complement Alternat Med 2013;2013:343594 [Crossref] [PubMed]
- Pang GM, Li FX, Yan Y, et al. Herbal medicine in the treatment of patients with type 2 diabetes mellitus. Chin Med J (Engl) 2019;132:78-85. [Crossref] [PubMed]
- Chang CL, Lin Y, Bartolome AP, et al. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med 2013;2013:378657 [Crossref] [PubMed]
- Meng B, Li J, Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr Pharm Des 2013;19:2101-13. [PubMed]
- Sholikhah EN. Indonesian medicinal plants as sources of secondary metabolites for pharmaceutical industry. J Med Sci 2016;48:226-39.
- Rohman A, Widodo H, Lukitaningsih E, et al. Review on in vitro antioxidant activities of Curcuma species commonly used as herbal components in Indonesia. Food Res 2019;4:286-93. [Crossref]
- Nihayati E, Wardiyati T, Retnowati R, et al. The curcumin content of temulawak (Curcuma xanthorriza Roxb.) rhizome as affected by N, K and micronutrients B, Fe, Zn. Agrivita 2013;35:218-26. [Crossref]
- Yuandani Y, Yuliasmi S. Curcuminoids analysis in Curcuma mangga rhizomes. Asian J Pharm Clin Res 2018;11:129-31. [Crossref]
- Kusumawati I, Kurniawan KO, Rullyansyah S, et al. Anti-aging properties of Curcuma heyneana Valeton & Zipj: A scientific approach to its use in Javanese tradition. J Ethnopharmacol 2018;225:64-70. [Crossref] [PubMed]
- Wahyuni DSC, Artanti AN, Rinanto Y. Quantitative analysis of Curcuminoid collected from different location in Indonesia by TLC-Densitometry and its antioxidant capacity. IOP Conf Ser Mater Sci Eng 2018;349:012015.
- Tanvir EM, Hossen MS, Hossain MF, et al. Antioxidant properties of popular turmeric (Curcuma longa) varieties from Bangladesh. J Food Qual 2017;2017:8471785 [Crossref]
- Zheng QT, Yang ZH, Yu LY, et al. Synthesis and antioxidant activity of curcumin analogs. J Asian Nat Prod Res 2017;19:489-503. [Crossref] [PubMed]
- Priyadarsini KI. Chemical and structural features influencing the biological activity of curcumin. Curr Pharm Des 2013;19:2093-100. [PubMed]
- Zhang DW, Fu M, Gao SH, et al. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med 2013;2013:636053 [Crossref] [PubMed]
- Stanić Z. Curcumin, a Compound from Natural Sources, a True Scientific Challenge - A Review. Plant Foods Hum Nutr 2017;72:1-12. [Crossref] [PubMed]
- Pothitirat W, Gritsanapan W. Quantitative analysis of curcumin, demethoxycurcumin and bisdemethoxycurcumin in the crude curcuminoid extract from Curcuma longa in Thailand by TLC-densitometry Mahidol Univ J Pharm Sci 2005;32:23-30.
- Badrunanto B, Wahyuni WT, Rafi M. Quantitative analysis of multi-components in Curcuma xanthorrhiza by single marker Indones J Pharm 2019;30:285-92.
- Lechtenberg M, Quandt B, Nahrstedt A. Quantitative determination of curcuminoids in Curcuma rhizomes and rapid differentiation of Curcuma domestica Val. and Curcuma xanthorrhiza Roxb. by capillary electrophoresis. Phytochem Anal 2004;15:152-8. [Crossref] [PubMed]
- Jayaprakasha GK, Rao LJ, Sakariah KK. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem 2006;98:720-4. [Crossref]
- Somparn P, Phisalaphong C, Nakornchai S, et al. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull 2007;30:74-8. [Crossref] [PubMed]
- Alam F, Shafique Z, Amjad ST, et al. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phytother Res 2019;33:41-54. [Crossref] [PubMed]
- Jhong CH, Riyaphan J, Lin SH, et al. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors 2015;41:242-51. [Crossref] [PubMed]
- Bansal P, Paul P, Mudgal J, et al. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp Toxicol Pathol 2012;64:651-8. [Crossref] [PubMed]
- Sarian MN, Ahmed QU, Mat So'ad SZ, et al. Antioxidant and Antidiabetic Effects of Flavonoids: A Structure-Activity Relationship Based Study. Biomed Res Int 2017;2017:8386065 [Crossref] [PubMed]
- Istriningsih E, Solikhati DIK. Aktivitas antidiabetik ekstrak rimpang kunyit (Curcuma domestica Val.) pada Zebrafish (Danio rerio). Parapemikir Jurnal Ilmiah Farmasi 2021;10:60-5.
- Widyowati R, Agil M. Chemical Constituents and Bioactivities of Several Indonesian Plants Typically Used in Jamu. Chem Pharm Bull (Tokyo) 2018;66:506-18. [Crossref] [PubMed]
- Andrie M, Taurina W, Ayunda R. Activities test of “jamu gendong kunyit asam” (Curcuma domestica Val.; Tamarindus indica L.) as an antidiabetic in streptozotocin-induced rats. Trad Med J 2014;19:95-102.
- Widowati W, Wargasetia TL, Afifah E, et al. Antioxidant and antidiabetic potential of Curcuma longa and its compounds. Asian J Agr Biol 2018;6:149-61.
- Widowati W, Sardjono CT, Wijaya L, et al. Free radicals scavenging activities of spices and curcumin. Proceedings of the Second International Symposium on Temulawak 2011;178-81.
- Lukiati B, Aulanni’am A, Darmanto W. The effects of Curcuma heyneana ethanolic extract on the superoxide dismutase activity and histological pancreas of type 1 diabetes mellitus rats. Int J Basic Appl Sci 2012;12:22-30.
- Chairani N, Ichwan M, Widyawati T. Effect of ethanol extract of temu giring (Curcuma heyneana Val.) rhizomes in reducing blood glucose level of mice after maltose loading. IOSR J Dent Med Sci 2020;19:55-61.
- Kim MB, Kim C, Song Y, et al. Antihyperglycemic and anti-inflammatory effects of standardized Curcuma xanthorrhiza Roxb. extract and its active compound xanthorrhizol in high-fat diet-induced obese mice. Evid Based Complement Alternat Med 2014;2014:205915 [Crossref] [PubMed]
- Santoso BSA, Sudarsono S, Nugroho AE, et al. Hypoglycemic activity and pancreas protection of combination of Morinda citrifolia Linn. juice and Curcuma xanthorrhiza Roxb. juice on streptozotocin induced diabetic rats. Indones J Pharm 2018;29:16-22. [Crossref]
- Cahyani MN, Pengaruh ekstrak temulawak (Curcuma xanthorrhiza Roxb.) terhadap kadar glukosa darah tikus wistar yang diinduksi aloksan. Thesis Fakultas Kedokteran: Universitas Tanjungpura; 2014.
- Adnyana IK, Yuliet EY, Kurniati NF. Antidiabetic activity of aqueous leaf extracts of Guazuma ulmifolia Lamk., ethanolic extracts of Curcuma xanthorrhiza and their combinations in alloxan-induced diabetic mice. Res J Med Plant 2013;7:158-64. [Crossref]
- Rahmayani I, Ambarsari L, Safithri M. Antihyperglicemic activity of Curcuma xanthorrizha Roxb. nanocurcuminoid emulsion on streptozotocin induced sprague-dawley rat. Curr Biochem 2016;3:66-79.
- Fitrianda E, Erniwati E. Aktivitas anti diabetes dan anti dislipidemia dari kombinasi ekstrak buah rimbang (SolanumtorvumSwartz) dan rimpang temulawak (Curcuma xanthorriza Roxb) pada mencit diabetes yang diinduksi aloksan. Scientia 2016;5:122-7.
- Pujimulyani D, Yulianto WA, Setyowati A, et al. Antidiabetic and antioxidant potential of Curcuma mangga Val extract and fractions. Asian J Agr Biol 2018;6:162-8.
- Pujimulyani D, Yulianto WA, Setyowati A, et al. Hypoglycemic Activity of Curcuma mangga Val. Extract via Modulation of GLUT4 and PPAR-γ mRNA Expression in 3T3-L1 Adipocytes. J Exp Pharmacol 2020;12:363-9. [Crossref] [PubMed]
- Pujimulyani D, Yulianto WA, Setyowati A, et al. Amylase inhibition and free radical scavenging activitites of white turmeric extract and fractions. Jurnal Teknologi dan Industri Pangan 2018;29:10-8. [Crossref]
- Moukette BM, Ama Moor VJ, Biapa Nya CP, et al. Antioxidant and Synergistic Antidiabetic Activities of a Three-Plant Preparation Used in Cameroon Folk Medicine. Int Sch Res Notices 2017;2017:9501675 [Crossref] [PubMed]
- Atal S, Atal S, Vyas S, et al. Bio-enhancing Effect of Piperine with Metformin on Lowering Blood Glucose Level in Alloxan Induced Diabetic Mice. Pharmacognosy Res 2016;8:56-60. [Crossref] [PubMed]
- Itokawa H, Shi Q, Akiyama T, et al. Recent advances in the investigation of curcuminoids. Chin Med 2008;3:11. [Crossref] [PubMed]
- Soni KB, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol 1992;36:273-5. [PubMed]
- Lao CD, Ruffin MT 4th, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 2006;6:10. [Crossref] [PubMed]
- Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 2001;21:2895-900. [PubMed]
- Vashisht T, Naidu CDM, Mandal C. Hypersensitivity reaction with metformin: a case report. Int J Basic Clin Pharmacol 2019;8:2763-5. [Crossref]
- Her C, Venier-Julienne MC, Roger E. Improvement of curcumin bioavailability for medical applications. Med Aromat Plants 2018;7:1000326 [Crossref]
- Nguyen TTH, Si J, Kang C, et al. Facile preparation of water soluble curcuminoids extracted from turmeric (Curcuma longa L.) powder by using steviol glucosides. Food Chem 2017;214:366-73. [Crossref] [PubMed]
- Rachmaniah O, Fazriyah LJ, Seftiyani NH, et al. Tailoring properties of acidic types of natural deep eutectics solvent (NADES): enhanced solubility of curcuminoids from Curcuma zeodaria. MATEC Web Conf 2018;156:01011.
Cite this article as: Priyangga KTA, Sagita CP, Yuliati L. A narrative review of curcuminoids from various Curcuma species in Indonesia as potential antidiabetic agents. Longhua Chin Med 2021;4:23.

