Traditional Chinese medicine, “Celastrol” and its nanotechnology for cancers: a narrative review
Introduction
Nanotechnology was a “technology on the nanoscale” identified in the late 1960s at ETH Zurich (1). It employs single atoms and molecules form functional structures to improve the chemical (2), physical, biological properties, processes, and phenomena of the materials (3). This includes the design, characterization, manufacture, shape, and size-controlled application of matters in the nanoscale (4).The size of nanoparticle system is ranged from a few nanometers (micelles) to several hundreds of nanometers (liposome) and the size of nanoscale protein material is often between 3 to 10 nanometers (nm) (5). Its nano-drug delivery system could interact with biomolecules locate inside or on the cell surface. The nanoparticle of the encapsulated drug would be delivered and penetrated into the cell. It could also be modified with fragments of antibodies or ligands, which targeting antigens or receptors on the cell surface for improving the specificity of drug delivery (6). The nano-drug delivery systems include organic nanoparticles such as nanoscale liposomes and micelles and inorganic nanoparticles such as gold or magnetic nanoparticles (7). Nanoparticles can penetrate the tissue system, facilitate cellular uptake of the drug, ensure action at the targeted location, and be affixed to the surface (8). This strategy applies to traditional Chinese medicine such as celastrol. In this mini-narrative review, we discuss the background of traditional Chinese medicine, “Celastrol” and its mechanisms of the nano-system for cancers as well as the toxicity and cancer targeting agent. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/lcm-20-48).
Methods
This mini-review summarized the articles on celastrol in nanotechnology for treating cancers through some library search engines such as SCI/SCIE, PubMed, and Scopus for at least 30
Background of celastrol
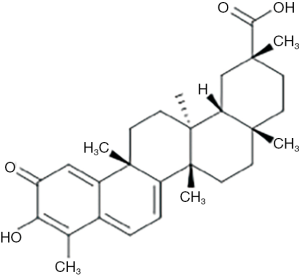
Celastrol is a pentacyclic triterpenoid, belongs to the family of quinone methides. Its formula C29H38O4 (Figure 1) with a molecular weight of 450.619 gmol−1, isolated from the root extracts of Tripterygium wilfordii and used to treat chronic inflammatory and autoimmune diseases (9). Celastrol induces apoptosis in various cancer cell lines via inhibition of inhibitor of kappa B kinase (IKK) (10), proteasome (11), topoisomerase activity (12), vascular endothelial growth factor (VEGF) receptor expression (13), and induction of heat shock proteins (14).
Accumulating evidence indicated that celastrol owns therapeutic potentials and diverse biological activities, including anti-inflammatory and anticancer properties. Celastrol inhibits swelling recurrence up to 55.25% when 10 mg/kg/day is used. It decreases immune cell filtration and proliferation into the synovial membrane, leading to decrease swelling, bone destruction as well as prevent inflammation. Prostate tumor weight is reduced by approximately 73% after administration of celastrol at 2 mg/kg/day for 16 days (15). However, its poor water solubility (13.25±0.83 mg/mL at 37 °C) (16), low bioavailability (17.06%) in the oral administration (17,18), and poor tumor selection represent major pitfalls for its clinical applications.
Mechanisms
In general, celastrol inhibits cell proliferation and induces cell apoptosis in tumors (19). It acts as a natural inhibitor of proteasome for regulating the activity of NF-κB. The pro-apoptotic protein Bax degrade the misfolding intracellular proteins because NF-κB transcription contributed to the cell migration, cell apoptosis as well as cell cycle progression and this is also one of the important factors for oncogenesis (20,21). By the deactivation of NF-κB activity, it would be influenced the level of proteasome leading to cell deaths or cancers. Thus, celastrol is an inhibitor for NF-κBtranscription. Besides, celastrol blocks the JAK/STAT signaling pathway by reducing the levels of cytokines or growth hormones that trigger JAK/STAT protein activation. It inhibits STAT3 phosphorylation and STAT3-mediates IL-17 expression, and T-helper 17 (Th17) differentiation and proliferation in multiple myeloma cells (22).
Nano-system
The efficacy of a nano-system in drug delivery depends on the size, shape, and other inherent biophysical or chemical characteristics. Polymeric nanomaterials act as carriers that exhibit high biocompatibility and biodegradability properties, various synthetic polymers such as polyvinyl alcohol, poly-
Growing studies showed that nanoparticle encapsulation improved the solubility of active components because the surface area increases and consequently its dissolution rate to have better bioavailability such as triptolide-loaded nanoparticles changed the solubility of triptolide, controlled its release, realized the target delivery of triptolide, and avoided the toxicity at non-target sites (24). Triptolide was another active component from Tripterygium wilfordii. Its cytotoxicity similar to the celastrol. However, celastrol is better in term of tolerance and efficacy in cancer since it was more soluble in water when combined the usage of nanotechnology (25). The as-prepared berberine-loaded chitosan nanoparticles were prepared and investigated the characteristics of in vitro release. Its encapsulation ratio of berberine-loaded chitosan nanoparticles and the total drug release degree are 65.4%±0.7% and 56.8%±1.7% respectively (26).
There are several nanosystems for celastrol that have been reported including: (I) The celastrol nanoparticle is modified to amphipathic molecules for enhancing the passive targeting effect on tumor through absorption and metabolism. Polyethylene glycol (PEG) has been introduced to make the celastrol dissolve in water easily (27). (II) Celastrol-loaded poly(ethylene glycol)-block-poly(ɛ-caprolactone) nanopolymeric micelles were also developed to improve the hydrophilicity of celastrol and PEGylated polyaminoacid-capped celastrol-loaded mesoporous silica nanoparticles (CMSN-PEG) to control the in vitro drug release behavior which exhibited high cytotoxicity in different cancer cells (28). (III) Axitinib (AXT) and celastrol (CST) combination nanoparticles (ACML) with CST loaded in the mesoporous silica nanoparticles (MSN) and AXT in PEGylated lipidic bilayers showed effective inhibition on angiogenesis and mitochondrial function. It’s efficiently internalized in SCC-7, BT-474, and SH-SY5Y cells (29-31).
These celastrol nanoparticles increased water solubility and cellular uptake (32-34). Most of the studies focused on intra-peritoneal injection and oral administration (35-38). It mainly focused on the solubility, cellular uptake, and in vitro drug release behavior of celastrol nanoparticles.
Toxicity
The toxicity of several types of celastrol nanoparticles are based on physicochemical properties such as interaction within the cells and the size of nanoparticles are related to the cytotoxicity. Smaller nanoparticles have a large surface area and penetrate the cells easily lead to cellular damage (39). Particle surface charges are another factor affecting the cellular uptake of nanoparticles. It's correlated to cytotoxicity because of the interaction between the cell organelles and their biomolecules (40). The stronger the electrostatic attraction, the more likely is the nanoparticles are to be internalized and would be damaged the other molecules through surface charges (41). Different shapes of the nanoparticles are also influenced by their toxicity as they generate different levels of reactive oxygen species (ROS) at the active sites in the cells for specific functions (42).
Cancer targeting agent
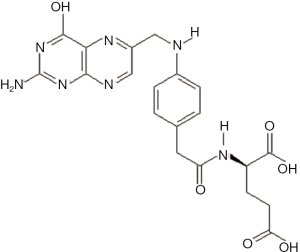
Basically, folic acid (Figure 2) or folate (pteroylglutamate) is water-soluble and often used as a targeting agent that can deliver celastrol selectively to cancer cells with overexpression of folate receptor on the surface (43,44). Folate receptor undergoes endocytosis within tumors. When the nano-carrier is passively targeted to tumors, it remains within the tumor or it diffuses out of the tumor and back into the bloodstream due to the high interstitial pressure within solid tumors and random diffusion. Folate modification makes the nano-carriers achieve a greater affinity in the tumor. The nano-conjugate is internalized by the folate-receptor via an endocytic pathway and is transported to an endosome or lysosome by intracellular vesicle transport.
Caveolae is small (approx. within 50 nm in diameter) flask-shaped pits in the membrane that resembles the shape of a cave. It constitutes up to the plasma membrane and uptake extracellular molecules via the specifically mediated folate-receptor by potocytosis that uses caveolae vesicles to bring molecules of various sizes into the cell and released into the cytosol (45). This specific binding of folic acid to folate receptor on cancer cells. For instant, folic acid-modified Doxorubicin nanoparticles (Dox-PLD-FA) showed a specific target to cancer cells, which overexpress the folate receptor (FR) (46).
Conclusions
Nanotechnology serves as an efficient tool to make celastrol into nanoscale and modify its physical properties. Incorporation of celastrol in the nano-system helps to increase solubility and stability, avoid toxicity, enhance pharmacological activity, improve tissue distribution, sustain delivery, and protect from physical or chemical degradation.
Some nano-systems for celastrol were developed such as polyethylene glycol (PEG) celastrol, celastrol-loaded poly(ethylene glycol)-block-poly(ɛ-caprolactone) nano-polymeric micelles, and the combination of axitinib (AXT) with celastrol nanoparticles in the mesoporous silica. Recently, folic acid-modified Doxorubicin nanoparticles (Dox-PLD-FA) are designed and showed a specific target to cancer cells compared with the other nano-systems. Hopefully, nano-systems of celastrol would be further developed in the future to improve its therapeutic efficacy.
Acknowledgments
Thanks to Prof. Chuanshan Xu and Prof. Albert Wingnang Leung for my PhD support.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/lcm-20-48
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/lcm-20-48). The authors have no conflicts of interest to declare. This manuscript is part of the Mr. Siukan Law, 2019 PhD in Chinese medicine thesis (CUHK).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kreuter J. Nanoparticles--a historical perspective. Int J Pharm 2007;331:1-10. [Crossref] [PubMed]
- Kaehler T. Nanotechnology: basic concepts and definitions. Clin Chem 1994;40:1797-9. [Crossref] [PubMed]
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 2004;104:293-346. [Crossref] [PubMed]
- Rao CNR, Cheetham AK. Science and technology of nanomaterials: current status and future prospects. J Mater Chem 2001;11:2887-94. [Crossref]
- Patrick Boisseau, Bertrand Loubaton. Nanomedicine, nanotechnology in medicine. C.R. Physique 2011;12:620-36. [Crossref]
- Bogunia-Kubik K, Sugisaka M. From molecular biology to nanotechnology and nanomedicine. Biosystems 2002;65:123-38. [Crossref] [PubMed]
- Mirza AZ, Siddiqui FA. Nanomedicine and drug delivery: a mini review. Int Nano Lett 2014;4:94. [Crossref]
- Wang N, Feng Y. Elaborating the role of natural products-induced autophagy in cancer treatment: achievements and artifacts in the state of the art. Biomed Res Int 2015;2015:934207 [Crossref] [PubMed]
- Fayaz AM, Girilal M, Venkatesan R, et al. Biosynthesis of anisotropic gold nanoparticles using Maduca longifolia extract and their potential in infrared absorption. Colloids Surf B Biointerfaces 2011;88:287-91. [Crossref] [PubMed]
- Jayaseelan C, Ramkumar R, Rahuman AA, et al. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind Crop Prod 2013;45:423-9. [Crossref]
- Walcott SE, Heikkila JJ. Celastrol can inhibit proteasome activity and upregulate the expression of heat shock protein genes, hsp30 and hsp70, in Xenopus laevis A6 cells. Comp Biochem Physiol A Mol Integr Physiol 2010;156:285-93. [Crossref] [PubMed]
- Lee JH, Koo TH, Yoon H, et al. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol 2006;72:1311-21. [Crossref] [PubMed]
- Yang H, Chen D, Cui QC, et al. Celastrol, a triterpene extracted from the Chinese "Thunder of God Vine," is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res 2006;66:4758-65. [Crossref] [PubMed]
- Venkatesha SH, Moudgil KD. Celastrol and its role in controlling chronic diseases. Anti-inflammatory nutraceuticals and chronic diseases. USA: Springer, 2016:267-89.
- Ng SW, Chan Y, Chellappan DK, et al. Molecular modulators of celastrol as the keystones for its diverse pharmacological activities. Biomed Pharmacother 2019;109:1785-92. [Crossref] [PubMed]
- Qi X, Qin J, Ma N, et al. Solid self-microemulsifying dispersible tablets of celastrol: formulation development, charaterization and bioavailability evaluation. Int J Pharm 2014;472:40-7. [Crossref] [PubMed]
- Li Z, Yao L, Li J, et al. Celastrol nanoparticles inhibit corneal neovascularization induced by suturing in rats. Int J Nanomedicine 2012;7:1163-73. [PubMed]
- Zhang J, Li CY, Xu MJ, et al. Oral bioavailability and gender-related pharmacokinetics of celastrol following administration of pure celastrol and its related tablets in rats. J Ethnopharmacol 2012;144:195-200. [Crossref] [PubMed]
- Wang XN, Wu Q, Yang X, et al. Effects of Celastrol on growth inhibition of U937 leukemia cells through the regulation of the Notch1/NF-kappaB signaling pathway in vitro. Chin J Cancer 2010;29:385-90. [Crossref] [PubMed]
- Zhou LL, Lin ZX, Fung KP, et al. Celastrol-induced apoptosis in human HaCaT keratinocytes involves the inhibition of NF-κB activity. Eur J Pharmacol 2011;670:399-408. [Crossref] [PubMed]
- Yang H, Chen D, Cui QC, et al. Celastrol, a triterpene extracted from the Chinese "Thunder of God Vine," is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res 2006;66:4758-65. [Crossref] [PubMed]
- Bose S, Banerjee S, Mondal A, et al. Targeting the JAK/STAT Signaling Pathway Using Phytocompounds for Cancer Prevention and Therapy. Cells 2020;9:1451. [Crossref] [PubMed]
- Krauel K, Pitaksuteepong T, Davies NM, et al. Entrapment of bioactive molecules in poly (alkylcyanoacrylate) nanoparticles. Am J Drug Deliv 2004;2:251-9. [Crossref]
- Xiong FL, Chen HB, Chang XL, et al. Research progress of triptolide-loaded nanoparticles delivery systems. Conf Proc IEEE Eng Med Biol Soc 2005;2005:4966-9. [Crossref] [PubMed]
- Xu W, Xing FJ, Dong K, et al. Application of traditional Chinese medicine preparation in targeting drug delivery system. Drug Deliv 2015;22:258-65. [Crossref] [PubMed]
- Lin AH, Li HY, Liu YM, et al. Preparation and release characteristics of berberine chitosan nanoparticles in vitro. China Pharm 2007;18:755-6.
- Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2003;2:214-21. [Crossref] [PubMed]
- Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discov Today 2005;10:1451-8. [Crossref] [PubMed]
- Bettina B, Masoud G, Fengfu L, et al. Controlled release of acyclovir through bioengineered corneal implants with silica nanoparticle carriers. J Tissue Eng Regen Med 2010;3:10-7. [Crossref]
- Choi JY, Gupta B, Ramasamy T, et al. PEGylated polyaminoacid-capped mesoporous silica nanoparticles for mitochondria-targeted delivery of celastrol in solid tumors. Colloids Surf B Biointerfaces 2018;165:56-66. [Crossref] [PubMed]
- Choi JY, Ramasamy T, Kim SY, et al. PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapy. Acta Biomater 2016;39:94-105. [Crossref] [PubMed]
- Nagase M, Oto J, Sugiyama S, et al. Apoptosis induction in HL-60 cells and inhibition of topoisomerase II by triterpene celastrol. Biosci Biotechnol Biochem 2003;67:1883-7. [Crossref] [PubMed]
- Li Z, Yao L, Li J, et al. Celastrol nanoparticles inhibit corneal neovascularization induced by suturing in rats. Int J Nanomedicine 2012;7:1163-73. [PubMed]
- Ding B, Wahid MA, Wang Z, et al. Triptolide and celastrol loaded silk fibroin nanoparticles show synergistic effect against human pancreatic cancer cells. Nanoscale 2017;9:11739-53. [Crossref] [PubMed]
- Niemelä E, Desai D, Nkizinkiko Y, et al. Sugar-decorated mesoporous silica nanoparticles as delivery vehicles for the poorly soluble drug celastrol enables targeted induction of apoptosis in cancer cells. Eur J Pharm Biopharm 2015;96:11-21. [Crossref] [PubMed]
- Lin Y, Yang X, Lu M, et al. Herbal compound triptolide synergistically enhanced antitumor activity of vasostatin120-180. Anticancer Drugs 2013;24:945-57. [Crossref] [PubMed]
- Peng X, Wang J, Li X, et al. Targeting Mast Cells and Basophils with Anti-FcεRIα Fab-Conjugated Celastrol-Loaded Micelles Suppresses Allergic Inflammation. J Biomed Nanotechnol 2015;11:2286-99. [Crossref] [PubMed]
- Yuan L, Liu C, Chen Y, et al. Antitumor activity of tripterine via cell-penetrating peptide-coated nanostructured lipid carriers in a prostate cancer model. Int J Nanomedicine 2013;8:4339-50. [PubMed]
- Nunes CT, Miners KL, Dolton G, et al. A novel tumor antigen derived from enhanced degradation of bax protein in human cancers. Cancer Res 2011;71:5435-44. [Crossref] [PubMed]
- Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest 2001;107:241-6. [Crossref] [PubMed]
- Jiang J, Oberdörster G, Elder A, et al. Does Nanoparticle Activity Depend upon Size and Crystal Phase? Nanotoxicology 2008;2:33-42. [Crossref] [PubMed]
- Kai W, Xiaojun X, Ximing P, et al. Cytotoxic effects and the mechanism of three types of magnetic nanoparticles on human hepatoma BEL-7402 cells. Nanoscale Res Lett 2011;6:480. [Crossref] [PubMed]
- Dreaden EC, Alkilany AM, Huang X, et al. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev 2012;41:2740-79. [Crossref] [PubMed]
- Mansoori GA, Mohazzabi P, McCormack P, et al. Nanotechnology in cancer prevention, detection and treatment: bright future lies ahead. WRSTSD 2007;4:226-57. [Crossref]
- Mansoori GA, Brandenburg KS, Shakeri-Zadeh A. A comparative study of two folate-conjugated gold nanoparticles for cancer nanotechnology applications. Cancers (Basel) 2010;2:1911-28. [Crossref] [PubMed]
- Scomparin A, Salmaso S, Eldar-Boock A, et al. A comparative study of folate receptor-targeted doxorubicin delivery systems: dosing regimens and therapeutic index. J Control Release 2015;208:106-20. [Crossref] [PubMed]
Cite this article as: Law S, Leung AW, Xu C. Traditional Chinese medicine, “Celastrol” and its nanotechnology for cancers: a narrative review. Longhua Chin Med 2021;4:27.