
Ren Shen Yang Rong Tang and other traditional Chinese medicines exhibit antioxidant and anti-inflammatory capacities and suppress acetylcholinesterase activity in PC12 neuronal cells
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder in elderly population worldwide. The pathology of AD is associated with extracellular amyloid beta (Aβ) deposition, intracellular tau accumulation, chronic inflammation, oxidative stress, loss of functional neurons and aging. In general, Aβ deposits are thought as the principal initiator of neuropathological development of AD (1-4). Aβ-formed plaques in the aging brain trigger the conversion of tau from a normal to a toxic state resulting in intraneuronal neurofibrillary tangles (5). The accumulation of Aβ plaques and tau tangles causes synaptic dysfunction and neuron loss (6) followed by chronic inflammation (neuroinflammation) and oxidative stress. The chronic neuroinflammation and oxidative stress conversely facilitate and exacerbate both Aβ plaques and tau tangles-induced pathological changes (7-9). As a result, functional neurons, especially cholinergic neurons undergo cell death that eventually causes brain atrophy, severe memory loss and behavioral deficits. During the past decades, despite extensive research and drug development targeting at Aβ plaques, little was achieved in clinical trials (10). The current drugs for AD treatment are mainly acetylcholinesterase (AChE) inhibitors (including donepezil, galantamine and rivastigmine) and memantine (a NMDA receptor antagonist) (11). Donepezil and other AChE inhibitors increase the function of cholinergic neurons through the inhibition of AChE. Memantine is used for AD patients who are intolerant of or have a contraindication to AChE inhibitors (12). However, both AChE inhibitors and memantine only temporarily ameliorate cognitive decline, but are unable to stop or reverse the progression of AD.
Traditional Chinese medicines [including Ren Shen Yang Rong Tang (RSYR), Shuan Zao Ren Tang (SZRT), Liu Wei Di Huang Wan (LWDH) and the herbs used in these formulas] have been used for improving the symptoms of neurodegenerative diseases in China, Japan, Korea and other Asian countries for more than 3,000 years (13-15). Among these medicines, RSYR and LWDH can improve aging-related frailty, memory impairment, chronic fatigue and feebleness. SZRT is known to cure insomnia with weakness. These Chinese medicines and the herbs used in their formulas have been demonstrated to increase cognitive function in elderly population (16). It has been reported that RSYR could promote proliferation of oligodendrocyte precursor cells from aged rat brain (17) and increase nerve growth factor (NGF) secretion in the cultured rat astrocytes (18). LWDH exhibited anti-inflammatory and antioxidant effects in obese rats (19). LWDH and its active fraction combination could improve the cognitive ability and neuronal synaptic function in aging or AD animal models through controlling the neuroendocrine immunomodulation network (20). SZRT showed positive effects on dementia patients with sleep disorders (21,22). However, little is known about the mechanisms of these herbal medicines on the improvement of AD. In the present study, we investigated antioxidant activity, cyclooxygenase-2 (COX-2) and AChE inhibitory activities of RSYR, SZRT, LWDH and 7 herbs by using ORAC (Oxygen Radical Absorbance Capacity) activity assay, COX-2 inhibitor screening assay and AChE activity assay. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/lcm-21-12).
Methods
Reagents
Extract powders of Ren Shen Yang Rong Tang (RSYR), Shuan Zao Ren Tang (SZRT), Liu Wei Di Huang Wan (LWDH), Huang Qi (Astragalus, HQ), Fu Ling (Poria cocos, FL), Dan Pi (Moutan cortex radicis, DP), Shan Yao (Dioscoreae rhizome, SY), Ze Xie (Alisma orientale, ZX), Shan Zhu Yu (Cornus officinalis, SZY) and Di Huang (Rehmannia glutinosa, Jio, DH) were provided by TSUMURA & CO., Tokyo, Japan.
Preparation of solutions of Chinese medicines
The above-mentioned 10 Chinese medicines were dissolved in double distilled water (DDW) or ethanol at a concentration of 5 w/v% (50 mg/mL) and filtered through 0.22 µm filters. The solutions were kept at –20 °C until use.
ORAC assay
ORAC assay kit was purchased from Cell Biolabs Inc. USA. The experiments were performed as stated in the kit instructions. Briefly, Chinese medicine samples (0.1 mg/mL, 25 µL) or the antioxidant standard (trolox) and fluorescein (150 µL; 70 nmol/L final concentration) solutions were added into the wells of a microplate (black 96-well plates, Nunc™ black microwell, ThermoFisher Scientific, Tokyo, Japan). The mixtures were then reacted with AAPH solution and the fluorescence intensities which present the antioxidant activity [peroxyl radical (ROO) scavenging activity] were measured by a fluorescence microplate reader (SH-9000Lab, HITACHI, Tokyo, Japan) as same as our previous report (23).
COX-2 inhibitor screening assay
COX-2 (human) screening assay kit was purchased from Cayman Chemical, USA. The reaction was performed as stated in the kit instructions. Briefly, the 10 Chinese medicines were dissolved in DDW or ethanol at a concentration of 5 w/v% (50 mg/mL). A total of 10 µL of the Chinese medicines were mixed with the 100% initial activity samples (10 µL of Heme and 10 µL of COX-2 in 160 µL of the reaction buffer) and incubated for 10 min at 37 °C. The mixtures were further reacted with 10 µL of Arachidonic Acid (substrate) for 2 min to produce Prostaglandins (PGs). The enzyme catalysis was stopped by the saturated Stannous Chloride solution. The PGs from the Chinese medicine samples and 100% initial activity samples were quantified by ELISA. Samples and PG standards were reacted with the PG Screening AChE Tracer and ELISA Antiserum in Mouse Anti-Rabbit IgG Coated Plate for 18 h and then developed with Ellman’s Reagent. The plate was read at 405 nm with a microplate reader (SH-9000Lab, HITACHI, Tokyo, Japan).
Cell culture
PC12 (RCB 0009, RRID:CVCL_0481) cells was purchased from Riken Cell Bank, Ibaraki, Japan. Cells were cultivated in the maintenance medium [high glucose DMEM medium (043-30085, FUJIFILIM Wako Pure Chemical Co., Japan) supplemented with 10% FBS and 1% GlutaMAX (ThermoFisher Scientific, Tokyo, Japan)] at 37 °C in a CO2 incubator. The neuronal inductive medium was the maintenance medium supplemented with NGF (50 ng/mL) (SRP3015), Sigma-aldrich, Tokyo, Japan] (24).
Cell viability assay
Cell viability in PC12 cells were measured by PrestoBlue® Assay according to our previous report (25). PrestoBlue® (A13261) solution was purchased from ThermoFisher Scientific, Tokyo, Japan. The reaction produced fluorescence intensities (which present the cell viability) were measured on a microplate reader (SH‐9000Lab, HITACHI, Tokyo, Japan).
Acetylcholinesterase activity assay
Colorimetric determination of AchE activity in PC12 cells was measured by Ellman’s assay (26). Briefly, PC12 cells were seeded in 24-well plates (30,000 cells/well) and then differentiated into neuronal cells by NGF-treatment. At the end of cultivation, after being washed two times by PBS (–), PC12 cells were homogenized in cell lysis buffer (0.12 M NaCl, 0.2% Triton X-100, 1 mM EDTA, 50 mM HEPES; pH7.5) on ice for 10 seconds. Protein quantity of the cell lysate was determined with a protein quantification kit (Protein Quantification Kit-Rapid, Dojindo Molecular Technologies, Inc., Kumamoto, Japan). 10 µL of the cell lysate with the same amount of protein and AChE standards were incubated with 180 µL Ellman’s reagent [0.1 M phosphate buffer pH 7.4, 0.5 mM dithiobisnitro-benzoate (DTNB) (346-08551, FUJIFILIM Wako Pure Chemical Co., Osaka, Japan)] without substrate in a 96-well plate for 30 min at room temperature. Then 10 µL of 20 mM acetylthiocholine iodide (A5751, Sigma-aldrich, Tokyo, Japan) (the substrate) was added into the plate. After 10–30 min incubation, the absorbance (405 nm) was measured with a microplate reader (SH‐9000Lab, HITACHI, Tokyo, Japan).
To measure direct inhibition of the Chinese medicines and donepezil (045-32321, FUJIFILIM Wako Pure Chemical Co., Osaka, Japan.), AChE (1,000 µunit/mL) [acetylcholinesterase from Electrophorus electricus (electric eel), C3389, Sigma-aldrich, Tokyo, Japan] was reacted with them for 10 min and then incubated with Ellman’s reagent for 30 min. After further been reacted with the substrate for 30 min, the absorbance of the mixed solution was measured at 405 nm.
Statistical analysis
Statistical analysis was executed by GNU PSPP Statistical Analysis Software (version 0.8.2-gad9374) (https://www.gnu.org/software/pspp/) and EZAnalyze Excel-based tools (http://www.ezanalyze.com/)according to our previous report (27). One-way analysis of the variance was first performed. Then the Post Hoc tests (including Tukey’s test and Bonferroni Correction) were accomplished. P<0.05 indicates the statistical significance. More than 5 samples were in each experimental group.
Results
Antioxidative activity of the Chinese medicines
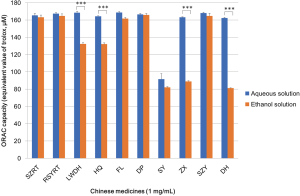
All 10 types of Chinese medicinal extracts powders showed good solubilities in both DDW and ethanol the same as the information provided by the manufacturer but left a small amount of sediment. We measured antioxidative activity of the 10 types of Chinese medicines by using ORAC assay. Figure 1 showed that, at a concentration of 1 mg/mL (0.1 w/v %), all 10 Chinese medicines showed peroxyl radical (ROO·) scavenging activity with equivalent value (80–160 µM) of the standard antioxidant trolox. Compared to the ethanol solutions, the aqueous solutions exhibited better ORAC capacity, especially DH, ZX, LWDH and HQ suggesting that the antioxidant compounds are mainly water-soluble molecules.
Potential anti-inflammatory activity of the Chinese medicines
We further tested if the Chinese medicines have anti-inflammatory activity by using ELISA-based COX-2 (human) inhibitor screening kit. As shown in Figure 2, most of the Chinese medicines in ethanol solution had a direct inhibition on COX-2 activity except LWDH and SZY. The inhibitory rates were 6.8–12.3%. We also tested COX-2 inhibition of aqueous solution of the Chinese medicines. The result showed that only SY, ZX and HQ had inhibitory effects on COX-2 activity. Although SY did not show high ORAC capacity, its aqueous solution exhibited the highest anti-COX-2 activity at an inhibition rate of 21.2%. The inhibition rates of SY and ZX in aqueous solutions were significantly higher than their ethanol solutions suggesting that their anti-inflammatory compounds are mainly water-soluble molecules.

Cytotoxicity of the Chinese medicines and donepezil
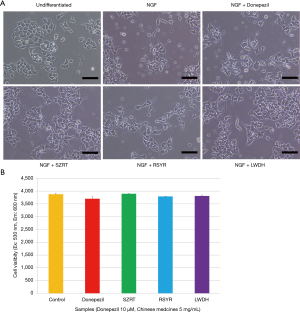
We then examines the effect of the Chinese medicines on intracellular AChE activity in NGF-differentiated PC12 neuronal cells. We first examined cytotoxicity of the Chinese medicines. Figure 3A showed that, after being cultivated with NGF (50 ng/mL) for 4 days, PC12 cells differentiated into neuron-like cells with long neurites as same as our previous report (17). When PC12 cells were treated with SZRT, RSYR and LWDH at a concentration of 5 mg/mL during NGF-differentiation, cell viability did not decrease suggesting that SZRT, RSYR and LWDH do not have cytotoxicity at 5 mg/mL. Donepezil at 10 µM (3.8 µg/mL) also did not show significant cytotoxicity although the cell viability decreased 5% of that in the control cells (Figure 3B). Therefore, we choose the Chinese medicines at 5 mg/mL and donepezil at 10 µM to perform the rest experiments.
Effect of the Chinese medicine on AchE activity in PC12 neuronal cells
AchE inhibitors, such as donepezil, have been used for improving the symptoms of dementia in clinical practice (28). We therefore tested if the Chinese medicines have the same effects. We first examined the direct inhibition of SZRT, RSYR, LWDH and donepezil on AChE activity. As shown in Figure 4A, after been incubated with donepezil, SZRT, RSYR and LWDH for 10 min, the residual activities of AChE (1,000 µunit/mL) were 14%, 74%, 72% and 60%, respectively. This data suggests that SZRT, RSYR and LWDH have some inhibitory effects on AChE in a cell free system.
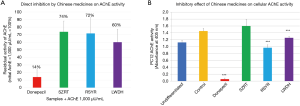
We then checked the inhibition of Chinese medicines on intracellular AChE in PC12 neuronal cells. We added donepezil (10 µM), SZRT, RSYR and LWDH (5 mg/mL) to PC12 cells with NGF (50 ng/mL) for 4 days. As shown in Figure 4B, compared to undifferentiated cells, the AChE activity of NGF-differentiated PC12 cells (the control cells) was increased 1.3 times. In donepezil-treated PC12 neuronal cells, the AchE activity was decreased to less than 10% of the control cells. Both RSYR and LWDH treatments also significantly inhibited AchE activity in PC12 neuronal cells to 66.7% and 86.4% of the control cells. However, SZRT did not inhibit the AChE activity of PC12 cells but slightly increased it to 110% of the control cells.
Discussion
Oxidative stress, chronic inflammation and functional neuron loss have been recognized as important contributors in the progression of AD (7-9). In the present study, we demonstrated that the 10 types of Chinese medicines, which have been used in clinical practice for improving the symptoms of AD, all exhibited excellent antioxidant activity, especially their aqueous solutions.
COX-2 is the inducible enzyme which initiates the inflammatory response by converting arachidonic acid into proinflammatory prostaglandins (mainly PGE2) and triggering production of other proinflammatory chemokines and cytokines (29). The inhibition of COX-2 is the key feature of steroids and nonsteroidal anti-inflammatory drugs. Figure 2 showed that most of the Chinese medicines (except LWDH and SZY) in their ethanol solutions exhibited COX-2 inhibition. Only aqueous solutions of HQ, SY and ZX displayed COX-2 inhibition. Interestingly, the COX-2 inhibitory rates of SY and ZX in their aqueous solutions were significantly higher than their ethanol solutions suggesting that the anti-inflammatory compounds of SY and ZX are mainly water soluble. Because ethanol solutions of the Chinese medicines also showed antioxidant and COX-2 inhibitory activities, extract powders of these Chinese medicines should be administrated as soil suspension. In that case, both water soluble and lipophilic compounds can be absorbed through the digest system.
The central cholinergic deficit is strongly related to mental function decline in AD. AchE inhibitors can increase the concentration of the neurotransmitter acetylcholine in the synaptic cleft and then improve the function of cholinergic neurons. Among AchE inhibitors, donepezil exhibits the best pharmacological profile in cognitive function, activities of daily living in AD and other types of dementia (30-32). However, the benefits of donepezil on AD patients are small and donepezil cannot stop the progress of AD. Our data confirmed that donepezil (10 µM) could markedly inhibit AChE activity in NGF-differentiated PC12 neuronal cells. Donepezil also has a direct inhibition on AChE activity in the cell free system. Both RSYR and LWDH also inhibited AChE activity in both cellular and cell free systems although they were not as efficacy as donepezil. However, donepezil showed cytotoxicity when the concentration was over 10 µM whereases the Chinese medicines did not show significant cytotoxicity even at a higher concentration (data not shown). Therefore, the Chinese medicines are safer. Although SZRT showed a direct inhibition on AChE activity as well as RSYR and LWDH, it did not inhibit but slightly increased the AChE activity in PC12 neuronal cells. It has been reported that SZRT could stimulate GABAA and serotonin receptors to effectively improve sleep quality and efficiency (33-35). It can be used for AD patients with sleep disorders.
Chinese medicines are known containing hundreds of bioactive compounds (36). Kobayashi et al. used a high performance liquid chromatograph (HPLC) to analyze the constituents in RSYR. Their results showed that RSYR contains ferulic acid, xanthotoxin, dehydropachymic acid, cinnamaldehyde, paeoniflorin, tenuifolin, flavonoids, formononetin, glycyrrhetic acid, liquiritin, gomisins and other organic compounds (17). Among these compounds, paeoniflorin reduces COX-2 expression and inflammatory reactions both in vivo and in vitro (37,38). Previous studies demonstrated that tenuifolin not only showed inhibitory activity on Aβ synthesis but also exhibited nootropic activity through inhibiting AChE and promoting norepinephrine and dopamine production (39-41). The main herb of RSYR, ginseng, has ginsenosides, saponins, flavonoids, polyphenols and other compounds. These bioactive compounds in ginseng showed antioxidant and anti-inflammatory effects in various in vitro and in vivo models (42,43). Ginsenosides also played a pronounced positive role in the prevention and treatment of AD and other neurological diseases (44). The main compounds of LWDH are 5-hydroxymethyl-2-furoic acid (HMFA), loganin and paeoniflorin. These compounds could be absorbed in to blood stream after oral administration (45) and exhibited antioxidant and anti-inflammatory activities (19,46). Moreover, LWDH has been demonstrated to against Aβ-induced paralysis in Caenorhabditis elegans through up-regulation of heat shock protein and its antioxidant activity (47). Suanzaoren, the major herb of SZRT, contains saligenin, saponin, flavonoids and sanjoinines. Flavonoids are believed to have various bioactive effects including anti-inflammatory, antioxidant, anti-aging, etc. Dietary flavonoids have been considered as a promising approach to prevent or slow the pathological development of neurodegenerative diseases (48). These evidences confirm our findings in the present study.
In conclusion, RSYR, LWDH, SZRT and the herbs used in their formulas have excellent antioxidant activity. The majority of them have inhibitory effects on COX-2 activity. RSYR and LWDH inhibit AChE activity in PC12 neuronal cells. They improve AD-associated pathogenic lesions probably partly through their inhibitory effects on oxidative stress, inflammation and AChE activity. However, more cellular and molecular studies are needed for future investigation.
Acknowledgments
The authors would like to thank Nathaniel Green’s proofreading.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/lcm-21-12
Data Sharing Statement: Available at http://dx.doi.org/10.21037/lcm-21-12
Peer Review File: Available at http://dx.doi.org/10.21037/lcm-21-12
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-21-12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval was not required for this study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer's disease. Nat Rev Neurosci 2007;8:499-509. [Crossref] [PubMed]
- Murphy MP, LeVine H 3rd. Alzheimer's disease and the amyloid-beta peptide. J Alzheimers Dis 2010;19:311-23. [Crossref] [PubMed]
- Sperling RA, Donohue MC, Raman R, et al. Association of Factors With Elevated Amyloid Burden in Clinically Normal Older Individuals. JAMA Neurol 2020;77:735-45. [Crossref] [PubMed]
- Insel PS, Donohue MC, Sperling R, et al. The A4 study: β-amyloid and cognition in 4432 cognitively unimpaired adults. Ann Clin Transl Neurol 2020;7:776-85. [Crossref] [PubMed]
- Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 2014;71:505-8. [Crossref] [PubMed]
- Pickett EK, Herrmann AG, McQueen J, et al. Amyloid Beta and Tau Cooperate to Cause Reversible Behavioral and Transcriptional Deficits in a Model of Alzheimer's Disease. Cell Rep 2019;29:3592-604.e5. [Crossref] [PubMed]
- Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer's disease. Biomark Med 2010;4:27-36. [Crossref] [PubMed]
- Chen Z, Zhong C. Oxidative stress in Alzheimer's disease. Neurosci Bull 2014;30:271-81. [Crossref] [PubMed]
- Kinney JW, Bemiller SM, Murtishaw AS, et al. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement (N Y) 2018;4:575-90. [Crossref] [PubMed]
- Lao K, Ji N, Zhang X, et al. Drug development for Alzheimer's disease J Drug Target 2019;27:164-73. review. [Crossref] [PubMed]
- Mehta M, Adem A, Sabbagh M. New acetylcholinesterase inhibitors for Alzheimer's disease. Int J Alzheimers Dis 2012;2012:728983 [Crossref] [PubMed]
- Mount C, Downton C. Alzheimer disease: progress or profit? Nat Med 2006;12:780-4. [Crossref] [PubMed]
- Park HJ, Kim DH, Park SJ, et al. Ginseng in traditional herbal prescriptions. J Ginseng Res 2012;36:225-41. [Crossref] [PubMed]
- Fang Z, Tang Y, Ying J, et al. Traditional Chinese medicine for anti-Alzheimer’s disease: berberine and evodiamine from Evodia rutaecarpa. Chin Med 2020;15:82. [Crossref] [PubMed]
- Sheng W, Wang Y, Li JB, et al. Clinical and Basic Research on Renshen Yangrong Decoction. Front Nutr 2019;6:175. [Crossref] [PubMed]
- May BH, Feng M, Zhou IW, et al. Memory Impairment, Dementia, and Alzheimer's Disease in Classical and Contemporary Traditional Chinese Medicine. J Altern Complement Med 2016;22:695-705. [Crossref] [PubMed]
- Kobayashi J, Seiwa C, Sakai T, et al. Effect of a traditional Chinese herbal medicine, Ren-Shen-Yang-Rong-Tang (Japanese name: Ninjin-Youei-To), on oligodendrocyte precursor cells from aged-rat brain. Int Immunopharmacol 2003;3:1027-19. [Crossref] [PubMed]
- Yabe T, Tuchida H, Kiyohara H, et al. Induction of NGF synthesis in astrocytes by onjisaponins of Polygala tenuifolia, constituents of kampo (Japanese herbal) medicine, Ninjin-yoei-to. Phytomedicine 2003;10:106-14. [Crossref] [PubMed]
- Perry B, Zhang J, Saleh T, et al. Liuwei Dihuang, a traditional Chinese herbal formula, suppresses chronic inflammation and oxidative stress in obese rats. J Integr Med 2014;12:447-54. [Crossref] [PubMed]
- Cheng X, Huang Y, Zhang Y, et al. LW-AFC, a new formula from the traditional Chinese medicine Liuwei Dihuang decoction, as a promising therapy for Alzheimer's disease: Pharmacological effects and mechanisms. Adv Pharmacol 2020;87:159-77. [Crossref] [PubMed]
- Lin SK, Tzeng JN, Lai JN. The core pattern of Chinese herbal formulae and drug-herb concurrent usage in patients with dementia. Medicine (Baltimore) 2019;98:e13931 [Crossref] [PubMed]
- Chen CJ, Liu X, Chiou JS, et al. Effects of Chinese herbal medicines on dementia risk in patients with sleep disorders in Taiwan. J Ethnopharmacol 2021;264:113267 [Crossref] [PubMed]
- Xiao L, Liao F, Ide R, et al. Enzyme-digested Colla Corii Asini (E'jiao) prevents hydrogen peroxide-induced cell death and accelerates amyloid beta clearance in neuronal-like PC12 cells. Neural Regen Res 2020;15:2270-2. [Crossref] [PubMed]
- Sakagami H, Suzuki R, Shirataki Y, et al. Re-evaluation of Culture Condition of PC12 and SH-SY5Y Cells Based on Growth Rate and Amino Acid Consumption. In Vivo 2017;31:1089-95. [PubMed]
- Xiao L, Mochizuki M, Nakahara T, et al. Hydrogen-Generating Silica Material Prevents UVA-ray-Induced Cellular Oxidative Stress, Cell Death, Collagen Loss and Melanogenesis in Human Cells and 3D Skin Equivalents. Antioxidants (Basel) 2021;10:76. [Crossref] [PubMed]
- Das A, Dikshit M, Nath C. Profile of acetylcholinesterase in brain areas of male and female rats of adult and old age. Life Sci 2001;68:1545-55. [Crossref] [PubMed]
- Xiao LI, Sakagami H, Miwa N. A New Method for Testing Filtration Efficiency of Mask Materials Under Sneeze-like Pressure. In Vivo 2020;34:1637-44. [Crossref] [PubMed]
- Sugimoto H, Yamanishi Y, Iimura Y, et al. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr Med Chem 2000;7:303-39. [Crossref] [PubMed]
- Chen C. COX-2's new role in inflammation. Nat Chem Biol 2010;6:401-2. [Crossref] [PubMed]
- Cacabelos R. Donepezil in Alzheimer's disease: From conventional trials to pharmacogenetics. Neuropsychiatr Dis Treat 2007;3:303-33. [PubMed]
- Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev 2018;6:CD001190 [Crossref] [PubMed]
- Black S, Román GC, Geldmacher DS, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke. 2003;34:2323-30. [Crossref] [PubMed]
- Yi PL, Tsai CH, Chen YC, et al. Gamma-aminobutyric acid (GABA) receptor mediates suanzaorentang, a traditional Chinese herb remedy, -induced sleep alteration. J Biomed Sci 2007;14:285-97. [Crossref] [PubMed]
- Yi PL, Lin CP, Tsai CH, et al. The involvement of serotonin receptors in suanzaorentang-induced sleep alteration. J Biomed Sci 2007;14:829-40. [Crossref] [PubMed]
- Chan YY, Chen YH, Yang SN, et al. Clinical Efficacy of Traditional Chinese Medicine, Suan Zao Ren Tang, for Sleep Disturbance during Methadone Maintenance: A Randomized, Double-Blind, Placebo-Controlled Trial. Evid Based Complement Alternat Med 2015;2015:710895 [Crossref] [PubMed]
- Ehrman TM, Barlow DJ, Hylands PJ. Phytochemical databases of Chinese herbal constituents and bioactive plant compounds with known target specificities. J Chem Inf Model 2007;47:254-63. [Crossref] [PubMed]
- Jia Z, He J. Paeoniflorin ameliorates rheumatoid arthritis in rat models through oxidative stress, inflammation and cyclooxygenase 2. Exp Ther Med 2016;11:655-9. [Crossref] [PubMed]
- Wu XX, Huang XL, Chen RR, et al. Paeoniflorin Prevents Intestinal Barrier Disruption and Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in Caco-2 Cell Monolayers. Inflammation. 2019;42:2215-25. [Crossref] [PubMed]
- Yoo KY, Park SY. Terpenoids as Potential Anti-Alzheimer’s Disease Therapeutics. Molecules 2012;17:3524-38. [Crossref] [PubMed]
- Lv J, Jia H, Jiang Y, et al. Tenuifolin, an extract derived from tenuigenin, inhibits amyloid-beta secretion in vitro. Acta Physiol (Oxf) 2009;196:419-25. [Crossref] [PubMed]
- Zhang H, Han T, Zhang L, et al. Effects of tenuifolin extracted from radix polygalae on learning and memory: a behavioral and biochemical study on aged and amnesic mice. Phytomedicine 2008;15:587-94. [Crossref] [PubMed]
- Xiong X, Huang G, Huang H. The antioxidant activities of phosphorylated polysaccharide from native ginseng. Int J Biol Macromol 2019;126:842-5. [Crossref] [PubMed]
- Chen F, Huang G. Antioxidant activity of polysaccharides from different sources of ginseng. Int J Biol Macromol 2019;125:906-8. [Crossref] [PubMed]
- Razgonova MP, Veselov VV, Zakharenko AM, et al. Panax ginseng components and the pathogenesis of Alzheimer's disease Mol Med Rep 2019;19:2975-98. (Review). [Crossref] [PubMed]
- Zhang N, Li L, Wang P, et al. Pharmacokinetics of the main compounds absorbed into blood after oral administration of Liu Wei Di Huang Wan, a typical combinatorial intervention of Chinese medical formula. J Nat Med 2013;67:36-41. [Crossref] [PubMed]
- Yan B, Sun G. Monitoring quality consistency of Liuwei Dihuang Pill by integrating the ultraviolet spectroscopic fingerprint, a multi-wavelength fusion fingerprint method, and antioxidant activities. J Sep Sci 2018;41:1182-91. [Crossref] [PubMed]
- Sangha JS, Sun X, Wally OS, et al. Liuwei Dihuang (LWDH), a traditional Chinese medicinal formula, protects against β-amyloid toxicity in transgenic Caenorhabditis elegans. PLoS One 2012;7:e43990 [Crossref] [PubMed]
- Calis Z, Mogulkoc R, Baltaci AK. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation. Mini Rev Med Chem 2020;20:1475-88. [Crossref] [PubMed]
Cite this article as: Xiao L, Liao F, Fan Y, Miwa N. Ren Shen Yang Rong Tang and other traditional Chinese medicines exhibit antioxidant and anti-inflammatory capacities and suppress acetylcholinesterase activity in PC12 neuronal cells. Longhua Chin Med 2021;4:13.

