An urge to improve the management in pharmacy of Chinese medicine: an overture of Good Pharmacy Practice (GPP) for pharmacy of Chinese medicine in Hong Kong
Introduction
Chinese medicine (CM) has been widely used in Hong Kong. CM involves acupuncture, moxibustion, and related disciplines such as Tuina Massage and Qi Gong and medication using CMs. Besides the common use of Western medicine (WM), CM is one of the most commonly used modes of treatment in Hong Kong (1).
Since various and stubborn diseases arise, accompanied by the swift development of the economy and society, the demand for CM and its pharmaceutical care services are increasing (2). While there has been growth in the number of pharmacy of CM (PCM), the concern for the quality and safety of pharmaceutical services is arising by the public. This quality and safety comprise the appropriateness of medication and a variety of services such as the pharmacy environment, medication adherence management, and even the pre- and post-prescribing management. In providing good CM pharmaceutical care services to patients, PCM need to provide standardized, reasonable guidelines to ensure the quality services (3).
Good Pharmacy Practice (GPP) is a critical standard in pharmacy setting for pharmacy services. GPP was first originated from the International Pharmaceutical Federation (FIP) in 1992. Then, in 1999, the joint FIP/World Health Organization (WHO) guideline on GPP was issued. The guideline’s objective is to improve and raise the standards of pharmacy services since the definition of GPP is “the practice of pharmacy that responds to the needs of the people who use the pharmacists’ services to provide optimal, evidence-based care. To support this practice it is essential that there will be an established national framework of quality standards and guidelines” (4). GPP is a set of national standards, and it guides specific achievable roles, functions, and activities, which execute the mission of pharmacy practice in the Western pharmacy. However, GPP is promoted for the pharmacy providing WMs only.
Existing regulatory regime for PCM in Hong Kong
Since the Chinese Medicine Ordinance (CMO) was passed and the regulation commenced in 1999 by the Chinese Medicine Council of Hong Kong (CMCHK) and the Department of Health (DH), the CM trader licensing system was introduced in 2008 to boost traders’ standard and safeguard public health. Under the licensing system, traders retailing or wholesaling Chinese herbal medicines must obtain a license from the Chinese Medicine Council’s Chinese Medicines Board (5).
In order to conduct retail business in consumer markets, including the sale or dispensing of any CMs specified in the schedules to the CMO, a retail license for the CM is required under the execution of the relevant legal provisions. Further, for the sale of toxic CMs (i.e., those listed in schedule 1 of the CMO), licensed CMs retailers must securely dispense them in accordance with the prescription from a registered CM practitioner (CMP) and record the information afterwards in compliance with the legal requirements and the “Practising Guideline for Retailers of Chinese Herbal Medicines” (Guideline). A person who violates those CMO regulations can be fined at level 6 in maximum and sentenced to 2 years in prison (5).
In addition, the Guideline states that CMs retailers who distribute CMs should assign one person to be responsible for supervising the proper dispensing of CMs and not more than two deputies. In terms of knowledge and experience, the nominees must meet these requirements as outlined in CMs regulation (CMR). While both the Ordinance and CMR do not specify qualification requirements for other employees, they do stipulate that these employees can only provide CMs under supervision from the designated persons. Any employee of a CMs retailer who breaches one of the relevant stipulations is legally liable and subject to disciplinary action. Moreover, the CMR and the “Practising Guideline for Retailers of Chinese Herbal Medicines” set out the dispensing practices of CMs retailers. These practices include checking prescriptions, packaging, dispensing, and decoction of Chinese herbal medicines (5).
Existing problems for the regulation for PCM in Hong Kong
Increase of the complaints regarding the licensed CMs retailers
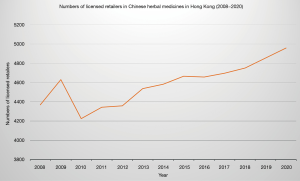
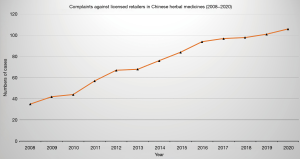
In Hong Kong, according to the statistical information from the CMCHK in 2020, the numbers of licensed retailers increased from 4,370 in 2008 to 4,958 in 2020 (Figure 1) (6). However, despite efforts to regulate CMs retailers in Hong Kong, reports of complaint cases have increased from 35 cases in 2008 to 106 cases in 2020 (Figure 2) (6). Most of the complaints are related to the “personnel” and “scope of business” aspects. “Personnel” refers to the persons (including responsible persons, dispensers and sales persons) that are engaged in the trade and they should possess the basic knowledge relevant to their duties and work conscientiously, under proper medicine shop management, to safeguard public health. And the “scope of business” includes the dispensing and sale of processed herbal medicines (such as verification of prescriptions, preparation, cross-checking, packaging and dispatching, and decoction of processed herbal medicines for customers), sale of single or multiple processed herbal medicines, purchase of processed herbal medicines, inspection, acceptance as well as the storage of processed herbal medicines and labelling; or the processing of herbal medicines according to business needs, preparing or compounding preparations for individual patients according to prescriptions given by CMPs and dispensing single CM granules for prescription (6).
Increasing trends of cases of poisoning related to the CMs
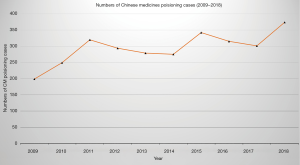
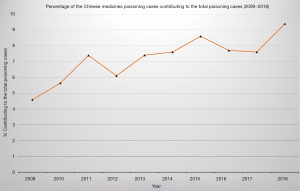
Each year since 2009, the Hong Kong Poison Information Centre (HKPIC) produces an annual report that summarizes all poisoning cases that the Centre received during the previous year. Based on the poisoning data of HKPIC between 2009 and 2018, one of the five commonest types of poisons was CMs which include both Chinese herbal medicines and proprietary CMs (pCM) (7). The number of CMs poisoning cases increased from 199 cases in 2009 to 374 cases in 2018 (Figure 3) (7-16). Concerning the percentage of the frequency of CMs poisoning cases occupying the total poisoning cases since 2009, there are significant proportion increased from 4.59% to 9.4% in 2018 (Figure 4) (7-16). For CMs poisoning, HKPIC concluded that three common injuries to our body were found including herb-induced liver injury, herb-related renal injury or aconite poisoning (17).
Low entrance requirement for managing PCM
Although both the CMs dispensers and the PCM in Hong Kong are regulated by the CMO and the “Practising Guideline for Retailers of Chinese Herbal Medicines”, the control of their practices are obscured. Under those regulations, a nominated person is responsible for the supervision of the dispensing of CMs and not more than two deputies shall be nominated, one of whom shall act in the absence of the said person. However, besides the qualified CMs professionals stated in Table 1 (A-D), for the basic requirement regarding knowledge and experience of the nominated person and his/her deputies, both a registered Western pharmacist with 1 year’ practical experience in dispensing CMs or anyone who has 5 years’ practical experience in dispensing CMs in Hong Kong are also satisfied with the regulation [Table 1 (E & F)] (18). CMs is known for being very comprehensive and complex in terms of quality, whether they are single drug prescriptions (prescriptions consisting of just one medicinal material) or compounded CMs (prescriptions combining two or more medicinal materials). Moreover, there is no legal registration for those CMs pharmacist (CMPharm) and their professionalism cannot be recognized.

Full table
Missing link for regulating the practice in PCM
Typically, crude drugs are used in decoction pieces and to manufacture pCM. Physical prerequisites for the use of crude medicines in clinics are safety and consistent quality. The Good Agricultural Practices (GAP) for cultivation of crude drugs are intended to control their quality throughout the production process and to guarantee their consistency, safety, and controllability. In Hong Kong, over 90% of the CMs are imported from China and most of the species are cultivated at the GAP bases (19). Therefore, the quality of the source of CMs can be controllable.
Moreover, in Hong Kong, the safety and consistent quality of pCM are officially assured by the implementation of Good Manufacturing Practice (GMP). GMP is a quality assurance system mainly adopted by the drug manufacturing industry globally. GMP ensures the entire production of drug products with the safe and consistent quality through establishing a series of standards for hardware and software during drug manufacturing, such as raw materials, equipment, premises, sanitation, labor and personnel training, and quality management. However, the GMP requirement in respect of pCM in Hong Kong is not mandatory. Under the GMP scheme, there are 27 out of 227 pCM manufacturers who have been awarded with GMP certificates (20).
Discussions
Notwithstanding there are different international standards applying to CMs such as GAP and GMP which are learning from WM in order to raise the safety and quality of the CMs and its products, there are no strict regulations for standardizing and regulating the practice in the PCM. GPP is one of the important measures to harmonize the dispensing activities and even the total management of PCM. The GPP guidelines are based on the pharmaceutical care given by pharmacists. The guidelines recommend that national standards are set for: the promotion of health, the supply of medicines, medical devices, patient self-care, and improving prescribing and medicine use by pharmacists’ activities. Nowadays, patient-centered services are provided by pharmacy intention for improving rational use of medications and finally enhancing the quality of life of patients (21). Therefore, improving and raising the pharmaceutical services’ standard in today’s rapidly changing healthcare system is important for the whole CM industry (22).
To face the strong competiveness of CM health services from other countries such as China, Taiwan and Japan, it is imperative for the PCM to advance the entire level of pharmaceutical services in Hong Kong. The capabilities of pharmaceutical service of PCM reflect a completeness of the CM policy and regulations including the development and supervision of PCM, the provision of professional talents, the development of standardized services, as well as the public’s cognition and recognition.
In view of the significant increase of the complaints and CMs poisoning cases, it indicates that poor management for PCM not only negatively affects safety and quality of the CMs but also lowers the image of the CM industry. Moreover, the high expectation of the public for the preventive measures, particularly focused risk communication with the CMs sellers, should be noticed by the regulatory authority. Otherwise, the uniformity, safety and effectiveness for dispensing practice of CMs cannot be guaranteed. In response to current issues stated above, GPP may be the ideal solution to tackle the problem through a rigid and practical regulation which plays an important role in the improvement of the current standard and services of the PCM in Hong Kong.
“Patient-centered” services from GPP can satisfy the patients’ needs
According to the definition of GPP from WHO and FIP, GPP focuses on four aspects in order to improve the pharmaceutical care on behalf of the patients (4). Currently, the increase of the patients’ complaints accompanying the growth of the PCM. It is shown that the existing pharmaceutical services are far from meeting the patients’ need. For example, most of the patients do not have clear and enough medical instructions before the administration of the CMs because of the short consultation time with the CMP and long waiting time for dispensing. Moreover, some PCM may not have stationed CMP, the nominated supervisor or his deputies may not explain in detail and guide the patients to take the CMs properly and thus the patients may take the CMs based on their own experience or in a wrong way. At the same time, it may also increase the chance of the adverse drug effect and poisoning by self-administration.
In order to address this phenomenon, it is recommended that it should set up a “CMPharm” who is the professional person in charge of the PCM so as to execute the GPP effectively. Through the roles and duties of the CMPharm, they plays an important function in different parts of GPP such as monitoring the dispensing process and the stock management. They should be responsible for the total management of the PCM. In the modern PCM, except the CMP providing the diagnosis services, the role of the CMPharm consists of being patient-centered, providing professional pharmaceutical services and ensuring the rational use and good quality of CMs. As a member of the medical team, the CMPharm is not only the provider of CMs, but also the reviewer of the CMPs’ prescriptions. At the same time, CMPharm is the professional person who collects and provides feedback on drug information; monitors and reports adverse drug reactions for patients. The professional knowledge of CMPharm can not only enhance the patient’s awareness and satisfaction with their pharmaceutical services, but also establish the image and reputation of PCM. If the solid execution of GPP can be carried out in the PCM, it can win the trust of the patients and enhance the modernization and competitiveness of PCM.
Whole procedures of dispensing and dispatching of CMs to patients can be controllable through GPP in order to minimize the risk of adulterations and poisonings
In Hong Kong, besides the considerable PCM and those that are operated by the non-governmental organization (NGO), most of the PCM are still under traditional management and certainly meet the minimum legal requirements. Nevertheless, due to the huge quantity and various CMs products existing on Hong Kong market, the adverse events of CMs usually occur. According to the reported adverse events of CMs in Hong Kong from 2013 to 2020 collected by the Chinese Medicine Regulatory Office (CMRO) of the DH, several species of CMs are usually involved in the adverse events (Table 2) (23). The four main causes of the cases are “overdose or long-term medication”, “misuse of alternative medicinal materials”, “adulteration of medicinal materials” and “improper preparation or decoction”. Obviously, these four aspects of incorrect use of CMs commonly happen which proves that the precise pharmaceutical care may not be successfully applied to those PCM because the experienced working staff may not be systematically trained with GPP. Ideally, PCM should provide consulting services on safe medication of CMs including the explanation of the pharmacological effects of the ingredients, the proper administration method, the appropriate dosage, the adverse reactions and side effects, the foods that should be avoided during medication. For some special cases, especially for those special patients (such as the elderly, children or pregnant women etc.) and those patients with specific diseases (such as diabetes, high blood pressure or other chronic diseases etc.), extra consulting time is needed to explain precautions and contraindications for medication in order to prevent the misuse and abuse of the CMs caused by the patients through self-administration. Therefore, PCM should act as a guard for providing patients with safe, effective, stable and appropriate CMs products.
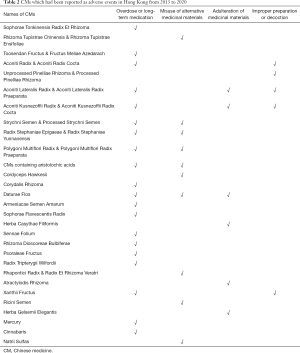
Full table
Establishment of the CMPharm for promoting GPP in the PCM
Nowadays, GPP currently is not compulsorily implemented in Hong Kong whereas GMP and GCP are promoted since the CMO had been launched. To be effectively executing GPP in Hong Kong, CMPharm is one of the important factors. As CMs specialists, CMPharm has been regarded as a trusted and accessible source for advice and treatment. In China, their role in health care is developing and growing to support patients in their use of CMs as well as assisting in clinical decision-making across a variety of specialties (24). CMPharm has the expertise to ensure that CMs are both chosen and used safely and effectively. In response to the demand of CM pharmaceutical services from the patients, the government or relevant authority should strictly regulate and monitor the PCM and develop the registration for the CMPharm. It is because traditionally most elderly CMs masters were not trained in the systematic way but only because of their experience and so it is difficult to give the confidence to the public. Therefore, through the establishment of CMPharm, GPP can be carried out by the CMPharm which enhance the confidence to the public when they go to PCM for prescribing CMs.
Finally, it is recommended that the relevant authorities conduct a research and develop a schedule for the execution of GPP in the CM industry. At the same time, the laws and regulations should be revised to create an environment for promoting the GPP. The coverage of GPP is integral to the PCM management so that it can guarantee the quality of service as well as the quality of CMs. Especially for the decoction pieces of CMs, different medicinal parts of the same plants have different applications and efficacy, so the medicinal ingredients and the mechanism of pharmacology are relatively complicated. If the CMs are unreasonably used by the patients, it will not only fail to give the expected treatment effect to the patient, but may also cause life-threatening results.
Conclusions
The adoption of GPP for enhancing the CM pharmaceutical care activities is an important step towards improving patient care and increasing their confidence. The implementation of GPP is the new direction for the development of CM industry because it can standardize and reinforce the pharmacies’ operations. Although the implementation of GPP in Hong Kong is still in its infancy, the GPP is the global trend for the pharmaceutical industry. Therefore, Hong Kong as an international city, should act as a leader for the promotion of GPP with the cooperation of all CM stakeholders in order to connect with the international standards.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-21-10). YF serves as an unpaid editorial board member of Longhua Chinese Medicine from Nov 2020 to Oct 2022. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chan K, Yeung HW. The progress of Chinese medicine in Hong Kong SAR China. In: Chan K, Lee H. editors. The Way Forward for Chinese Medicine. London and New York: Harwood Academic Publishers, 2002:261-90.
- Zhang LB, Zhang DY, Fu YL. International trends of development of traditional Chinese medicine. The Journal of Beijing Traditional Chinese Medical University 2007;30:149-52.
- Hong KR. Standardization of patient counseling and pharmacy practice. J Korean Soc Health-Syst Pharm 2004;21:343-56.
- World Health Organization. Joint FIP/WHO Guidelines on Good Pharmacy Practice: standards for quality of pharmacy services. WHO Technical Report Series. 2011. Available online: http://apps.who.int/medicinedocs/documents/s18676en/s18676en.pdf
- The Chinese Medicine Council of Hong Kong. Licensing of Chinese Medicines Traders. [updated and cited 18 Jan 2021]. Available online: https://www.cmchk.org.hk/pcm/eng/#main_down01.htm
- The Chinese Medicine Council of Hong Kong. Chinese Medicine Council of Hong Kong Annual Report (2008-2020). [updated and cited 18 Jan 2021]. Available online: https://www.cmchk.org.hk/pcm/eng/#main_public01.htm
- Chan YC, Tse ML, Lau FL. Hong Kong Poison Information Centre: Annual Report 2009. Hong Kong Journal of Emergency Medicine 2011;18:221-31. [Crossref]
- Chan YC, Tse ML, Lau FL. Hong Kong Poison Information Centre: Annual Report 2010. Hong Kong Journal of Emergency Medicine 2012;19:110-20. [Crossref]
- Chan YC, Tse ML, Lau FL. Hong Kong Poison Information Centre: Annual Report 2011. Hong Kong Journal of Emergency Medicine 2012;19:394-404. [Crossref]
- Chan YC, Tse ML, Lau FL. Hong Kong Poison Information Centre: Annual Report 2012. Hong Kong Journal of Emergency Medicine 2013;20:371-81. [Crossref]
- Chan YC, Tse ML, Lau FL. Hong Kong Poison Information Centre: Annual Report 2013. Hong Kong Journal of Emergency Medicine 2014;21:249-59. [Crossref]
- Chan YC, Tse ML, Lau FL. Hong Kong Poison Information Centre: Annual Report 2014. Hong Kong Journal of Emergency Medicine 2015;22:376-87. [Crossref]
- Chan YC, Tse ML, Lau FL. Hong Kong Poison Information Centre: Annual Report 2015. Hong Kong Journal of Emergency Medicine 2016;23:358-70. [Crossref]
- Chan YC, Chan CK, Ng CH, et al. Hong Kong Poison Information Centre: Annual Report 2016. Hong Kong Journal of Emergency Medicine 2017;24:244-54. [Crossref]
- Lau KK, Chow TYA, Chan CK, et al. Hong Kong Poison Information Centre: Annual report 2017. Hong Kong Journal of Emergency Medicine 2018;25:313-23. [Crossref]
- Chow TYA, Chan CK, Ng SH, Tse ML. Hong Kong Poison Information Centre: Annual report 2018. Hong Kong Journal of Emergency Medicine 2020;27:344-55. [Crossref]
- Ng F. Ten-year profile of acute poisoning patients presenting to an Accident and Emergency Department requiring intensive care in a regional hospital of Hong Kong. Hong Kong Journal of Emergency Medicine 2019;26:3-14. [Crossref]
- The Chinese Medicine Council of Hong Kong. Handbook of the Application for Chinese Medicines Trader Licences. [Jan 2021]. Available online: https://www.cmchk.org.hk/pcm/eng/#main_down01.htm
- Liu S, Wei JH, Chen SL. Study on criteria for GAP certification of wild tending species of Chinese crude drug. Chin Med J Res Pract 2005;7:1-4.
- The Chinese Medicine Council of Hong Kong. List of manufacturers of proprietary Chinese medicines. [Jan 2021]. Available online: https://www.cmchk.org.hk/pcm/eng/#main_list_2018.htm
- Sun Q, Santoro MA, Meng Q, et al. Pharmaceutical policy in China. Health Aff 2008;27:1042-50. [Crossref] [PubMed]
- Martins E. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 2014;4:177. [PubMed]
- The Chinese Medicine Regulatory Office of the Department of Health. A Reference Book for Chinese medicines which had been reported as adverse events in Hong Kong (Chinese version only). Available online: https://www.cmro.gov.hk/html/eng/health_info/res_publication.html
- Zhang XR. Traditional medicine with health care and the impact of globalization on protection of traditional medicine. Kuwait: The Seventh International Conference on the Impact of Globalization on Development and Health Care Services in Islamic Countries, 2002.
Cite this article as: Lam K, Feng Y. An urge to improve the management in pharmacy of Chinese medicine: an overture of Good Pharmacy Practice (GPP) for pharmacy of Chinese medicine in Hong Kong. Longhua Chin Med 2021;4:16.