The traditional Chinese medicine WuJiaPi (Acanthopanacis cortex) and its main anti-inflammatory terpenoids
Introduction
The shrubby plant Acanthopanax gracilistylus, also known as Eleutherococcus nodiflorus or Eleutherococcus gracilistylus (Araliaceae family), is largely used in traditional medicine (Figure 1). The plant is native to east Asia, and usually grows on the slopes of the mountains of China (notably in the Hubei, Henan, Anhui provinces), in Japan and a few other Asian countries. It is used to treat a number of diseases and conditions, such as rheumatism, arthritis in the limbs and knees, and to reduce pain in the lower back for examples. Medications are essentially prepared from dried slices of the bark of the roots which can be commonly found at many Asian markets and specialty stores. Some shops also propose powdered Acanthopanax bark, granules and different formulas including Acanthopanax bark, also called Acanthopanacis cortex (Pinyin name: Wu Jia Pi) combined with other herbs. According to the TCM principles, the Wu Jia Pi (or Wujiapi) medication is considered as warm, dry, pungent, and bitter. It is mostly used to strengthen bones and joints, to promote diuresis and to tonify the liver and kidneys.

The use of Wu Jia Pi is popular, but care should be taken to use the right plant to avoid risk of unwanted side effects or toxicities. There are reported cases of confusion between Acanthopanax root bark (WuJiaPi) and Periploca root bark (Xiang Jiapi), or confusion with other species such as Hedyotis hedyotidea and Acanthopanax giraldii (1). Cases of adulterant herbal medicines are not rare with this plant (2). The long and thick roots of A. gracilistylus (or A. sessiliflorus also used as a source of WuJiaPi) have a thick bark, fragrant smell and no duramen. They are usually dried in sunlight, cut into thick pieces, or powdered. The root pieces or powder can be used in decoctions or soaked in wine, to produce the so-called Wu Jia Pi Jiu (wine) used to increase circulation, to invigorate blood, and to relieve muscle pain (Figure 1). WuJiaPi liquor is an ancestral medicinal liquor originally recorded by Dr Shizhen Li (CE 1518-1593) in his famous book “Compendium of Materia Medica”. It contains a large diversity of natural products and a complex aroma profile (3).
Acanthopanacis cortex (root bark) is used in different traditional medicine recipes, in China (WuJiaPi), Japan (Gokahi), Korea (Ogapi) and other Asian countries (4). A classical method of preparation consists to add 1 g of Acanthopanacis cortex (powder) in 100 mL of distilled water at room temperature overnight and boiled for 60 min. The extract is filtrated to remove insoluble materials and then used. There are other similar procedures used to prepare the medication from Acanthopanacis cortex. For more than 50 years the chemical constituents of WuJiaPi have been investigated, leading to the identification of a large variety of natural products including many steroidal glycosides, lignans, flavonoids, cerebrosides, anthocyanins and other products (5-8). But remarkably, the plant is a rich source of triterpenoid saponins. The present review provides an updated survey of the diversity of natural products isolated from A. gracilistylus and Acanthopanax related species in recent years, with a specific focus on triterpenoid glycosides and their pharmacological properties.
Acanthopanax gracilistylus extracts
As mentioned above, Acanthopanacis cortex (Wu Jia Pi) is used in traditional Chinese medicine for a long time to dispel pathogenic wind and strengthen the body. Its use was first recorded in the text “Shen Nong Ben Cao Jing”, which dates back to 220−280 A.D. An analysis of the chloroplast genomes of different Araliaceae has revealed that the specie Eleutherococcus gracilistylus (synonym for Acanthopanax gracilistylus) is more closely related to E. trifoliatus than to E. senticosus and another specie called E. sessiliflorus (Siberian ginseng), at least from a phylogenic view (9). WuJiaPi extracts are usually prepared from the root bark of Acanthopanax gracilistylus or in some cases from A. senticosus. This latter plant is also classified as Eleutherococcus senticosus and the root preparations are called radix Acanthopanacis senticosus or Ciwujia (Figure 2).A comprehensive analysis of the ethnobotany, medicinal uses, chemical composition, pharmacological activity, and toxicology of this plant has been published recently (10). Here we will essentially focus on A. gracilistylus (WuJiaPi) and its chemical components.

A. gracilistylus is native to China but the plant can be cultivated in various parts of the world. Root extracts of the plant grown in Europe (Poland in particular) have revealed robust antioxidant activities, with a marked capacity to inhibit acetylcholinesterase associated with the presence of various phenolic compounds (11). The plant extract showed almost no cytotoxic activity toward HL-60 leukemia cells (or only at a high concentration, IC50 >800 µg/mL) which are usually quite sensitive to drugs (12). However, other studies have evidenced significant anti-proliferative and cell cycle blocking activities with A. gracilistylus extracts (13,14). Moreover, an extract of A. gracilistylus was found to exert an immunomodulating activity on human lymphocytes, enhancing monocyte function to produce cytokines, and suppressing the production of pro-inflammatory cytokines by lymphocytes in vitro (15). The anti-inflammatory action could be useful for different pathologies, for example for the treatment of postmenopausal osteoporosis. Treatment with an aqueous extract from Acanthopanacis cortex was found to increase bone mass and to decrease bone resorption [with down-regulation of the receptor activator of nuclear factor kappa-B ligand (RANKL)] in an experimental rat model of osteoporosis (16).
The marked anti-inflammatory action of Acanthopanacis cortex extracts encourages the use of TCM containing this plant, such as WuJiaPi but it also stimulates the development of modern medicines derived from this material. Flower-like gold nanoparticles (called gold nanoflowers) have been prepared using Acanthopanacis cortex extract and were found to inhibit the production of iNOS (inducible nitric oxide synthase) and cyclooxygenase-2 proteins as well as nitric oxide (NO) and prostaglandin E2 (17,18).
Bioactive components of Acanthopanax gracilistylus
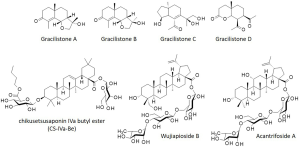
A. gracilistylus is a rich source of bioactive natural products. The first chemical components of the plant extracts were characterized in the 1960s (5,6) and diverse categories of molecules have been identified over the past 60 years (19). New compounds are regularly isolated and characterized. One of the most recent study concerned the identification of rare cyperane-type sesquiterpenoids, designated gracilistones C (two enantiomers) and the novel norsesquiterpenoid gracilistone D (Figure 3). This latter compound was found to potently inhibit the production of NO in lipopolysaccharide-induced in RAW 264.7 macrophages (20). The compound was apparently more potent than the eudesmane-type sesquiterpenoids gracilistones A and B previously identified (21). There are other bioactive terpenoids in A. gracilistylus such as the anticancer saponin chikusetsusaponin IVa butyl ester (CS-IVa-Be) which functions as an antagonist of IL-6 receptor (22) and the lupane-triterpene glycoside named wujiapiosides A and B (23,24). Wujiapioside B (also known as oplopanaxoside C or cirenshenoside H) bears a structural analogy with acantrifoside A (Figure 2) also found in A. gracilistylus and A. koreanum (24,25). Acantrifoside A shows anti-inflammatory effects, but it is a less potent compounds than acankoreosides A and B, discussed hereafter (26).
There are many other bioactive molecules in A. gracilistylus extracts, such as the compound called kaurane acid glycoside A isolated many years ago (19) and later found in the pericarp of the plant Datura metel (27). This compound has the capacity to promote the proliferation of vascular endothelial cells, and to reduce the expression of NFκB/p65 protein in a dose-dependent manner (27). Different kaurenoic acid-type diterpenoids can be found in the root bark of A. gracilistylus (28). Occasionally, eleutherosides can be found in A. gracilistylus extracts, such as eleutheroside D identified together with other phenylpropanoid and lignan glycosides (19).Otherwise, eleutherosides are essentially found in E. senticosus (Ciwujia) (29,30).
The sapogenin acankoreagenin
The most abundant glycosylated terpenoids in A. gracilistylus derive from the sapogenin acankoreagenin (ACK, Figure 4) which can be found in the roots but also in the leaves of the plant. It is a pentacyclic molecule isolated from the leaves of diverse plants, principally from the Acanthopanax family and a few other plants. The product is called acankoreagenin in different studies, or acankoreanogenin in the initial work when it was first isolated from A. gracilistylus (30,31) and later from A. trifoliatus (32). About forty years ago, the same compound designated 3α-hydroxy-lup-20(29)-ene-23,28-dioic acid was isolated from the leaves of another Araliaceae: Schefflera octophylla, used in Vietnamese folk medicine for the treatment of rheumatism and liver diseases (33). This plant is known to contain various triterpene glycosides and to display antinociceptive and anti-inflammatory activities (34-36).Later, another name was given to the same compound: HLEDA, for 3-Hydroxy-Lup-20(29) En-23-28-Dioic Acid which as revealed marked activities in different rat experimental models of gastric ulcer (37). HLEDA was isolated from Schefflera heptaphylla and revealed activity against the herpes simplex virus type 1 (HSV-1) (38). More recently, this compound HLEDA, which is in fact acankoreagenin (ACK), was found to inhibit the NF-κB signaling pathway, to reduce the secretion of the alarmin protein HMGB1 and to reduce the expression of pro-inflammatory cytokines in lipopolysaccharide-induced macrophages (39). Thus, the same compound has been named differently, acankoreagenin, acankoreanogenin, or HLEDA. It can be found in several Acanthopanax and Schefflera species (Figure 4), together with various glycoside derivatives. These plants and their extracts are frequently used in TCM for their anti-inflammatory properties (36,40).
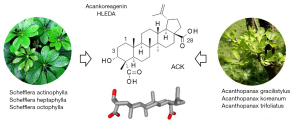
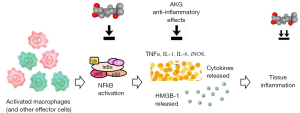
ACK is an anti-inflammatory compound, but its molecular targets are not well characterized. Different types of activities have been reported. The compound was found to inhibit the enzymes α-glucosidase, PTP1B (IC50 ~ 13 and 16 µM) and to a lower extent α-amylase (IC50 =31 µM) implicated in diabetes. ACK proved to be more efficient than acarbose used as a positive control for α-glucosidase and α-amylase inhibition, and more potent than ursolic acid used as a positive control for PTP1B inhibition (41). But the most remarkable effect is a drug-induced inhibition of the activation of the transcription factor NF-κB in rat insulinoma RIN-m5F β cells, associated with a reduction of the production of the protein iNOS (41). The anti-inflammatory activity of acankoreagenin has been well characterized also in a mice model of fulminant hepatitis. In this case, the compound was found to reduce the serum levels of inflammatory cytokines such as TNFα and IL-1β and to attenuate the release of the protein HMGB1 (42). The systemic release of HMGB1 is a major pro-inflammatory signal and a prominent damage-associated molecular pattern (DAMP) (43). Other models have been used to characterize the anti-inflammatory action of acankoreagenin notably using lipopolysaccharide-stimulated murine macrophages, inhibiting the release of several inflammatory mediators like iNOS, COX-2, TNFα and IL-6 (44) (Figure 5). ACK was found to attenuate mouse ear oedema (induced by tetradecanoylphorbol acetate) with an efficacy inferior to that of the positive control dexamethasone but the compound potently suppressed the production of proinflammatory mediators in this in vivo model (44).
Acankoreanogenin is not a cytotoxic compound but it can reduce the proliferation of cancer cells, alone or in combination with cytotoxic drugs. A synergistic action was found when combining ACK and the tubulin polymerization inhibitor docetaxel, to reduce the growth and migration of prostate cancer cells and to induce apoptotic cell death (45). Similarly, a synergy was observed with the nucleoside analog gemcitabine in Panc-1 pancreatic cancer cells (46). In both cases, the synergy was associated with the inhibition of the expression of proteins NF-κB and phospho-STAT3 (45,46).
The leaves of A. gracilistylus contain ACK and a 1-hydroxylated derivative which has been named acangraciligenin S (Figure 5). It is also an anti-inflammatory compound, inhibiting NO production in lipopolysaccharide-induced BV2 microglia but with a reduced efficacy compared to ACK (47). The plant also contains glycoside derivatives of these compounds, known as acankoreoside A and acangraciliside S (Figure 6).Another structural analogue of ACK is called impressic acid (Figure 6) is mainly found in A. koreanum. Both compounds are potent regulators or the NF-κB pathway. Like ACK (41), impressic acid can strongly inhibit NF-κBactivation, thereby reducing matrix protein degradation and cartilage degradation (48). This anti-inflammatory compound has been found to reduce the production of pro-inflammatory cytokines like TNFα, via an inhibition of NF-κB and PPARγ (peroxisome proliferator-activated receptor gamma) target genes (44,49,50). Impressic acid was found to activate the iNOS/NO pathway in endothelial cells (51). This compound thus appears functionally similar to ACK. Little or no significant differences between ACK and impressic acid were observed when comparing their anti-inflammatory effects (45,46), suggesting therefore that the carboxyl group at C-23 of ACK is not essential for the anti-inflammatory action. It possibly explains why some of the active glycoside derivatives of ACK, such as acankoreosides B and C, do not possess this carboxyl function (see below).
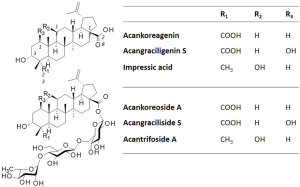
Acankoreosides: glycosides derivatives of acankoreagenin
Different glycosidic triterpenes have been isolated from A. gracilistylus such as the aforementioned compounds wujiapiosides A and B (22,23) and acangraciliside S (47) (Figure 6). The leaves of the plant are rich in lupane-triterpene glycosides, such as acantrifoside A which is a close analogue of acankoreosides A and B (23). Acantrifoside A has been isolated from the leaves and fruits A. gracilistylus but also from A. koreanum and A. trifoliatus (24,52,53). It has anti-inflammatory effects, but it is a less potent compound than the related acankoreosides at suppressing TNFα and IL 1β mRNA expression in lipopolysaccharide stimulated macrophages (25).
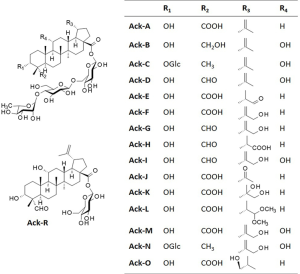
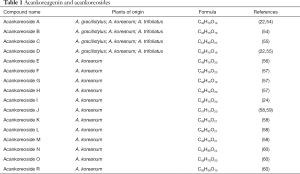
The main triterpenes glycosides found in A. gracilistylus are called acankoreosides (Ack), corresponding to a small group of 16 compounds isolated from various Acanthopanax species. All these compounds were primarily isolated from A. koreanum but some of them, such as Ack A-to-D were also found in A. gracilistylus and A. trifoliatus (Table 1).Apart from Ack-R which is an atypical derivative, all the other acankoreosides bear a trisaccharide unit α-L-Rham (1→4)-β-D-Glc(1→6)- β-D-Glc- at position C-28 and differ by the nature of the R1-R2-R3-R4 substituents on the aglycone (Figure 7). There are two bidesmosidic derivatives, Akt-C and Akt-N with a glucose unit at C-3 in addition to the trisaccharide at C-28. The other compounds are monodesmosides. They possess an acid function typical of ACK at the R2 position, or a methyl group as in impressic acid, or an alcohol group (Ack-B) or an aldehyde function for Ack-D, G, H and I. The R3 substituent at C-19 position is usually an isopropenyl group, more or less substituted. Half of the compounds have a hydrogen at R4 as in ACK, the other have a hydroxyl group as in impressic acid. Ack-R is an atypical compound in the series, with a monosaccharide (glucose) at C-28, not a trisaccharide unit (61). It was designated Ack-R but apparently the series does not include Ack-P and Ack-Q (not found in the literature). Ack-R is an analogue of Ack-D, with the same aglycone moiety but a different C-28 glycoside residue (Figure 7).
Full table
The anti-inflammatory activity of acankoreosides A, B and D, all three isolated from A. gracilistylus, has been compared. The three compounds have the capacity to reduce NFκB activity and the resulting production of the early inflammatory cytokines TNFα and IL 1β in stimulated macrophages. In addition, Ack-A and -B can suppress the secretion of the protein HMGB1 which is considered a late inflammatory cytokine. The most efficient compound is Ack-A, endowed with marked anti-inflammatory properties (25). It is believed to contribute largely to the anti-inflammatory action of Acanthopanax plant extracts and WuJiaPi in particular (24). Ack-A can be easily extracted from different plants and its content specifically quantified (40).
Conclusions
The Chinese medication WuJiaPi has been described in the Chinese literature since more than two thousand years. It is largely used as a Qi-tonifying agent and to treat various diseases and conditions. For examples, the preparation can be used to improve kidney function, to strengthen bones, or to alleviate lumbar disc herniation (62). It is largely used in China, in Japan and apparently also in Russia where it is called “Eleutherokokk koljucij”, although this name refers to Eleutherococcus senticosus, not to Eleutherococcus gracilistylus (Acanthopanax gracilistylus) (63). WuJiaPi is a rich source of natural products and many of them have been shown to display an anti-inflammatory action.
Different natural products likely contribute to the beneficial effects of WuJiaPi, in particular the various terpenoids evoked here. Surprisingly, acankoreagenin is anti-inflammatory agent rarely studied, and largely ignored compared to structurally related lupane triterpenoids, like betulinic acid. Betulinic acid, or 23-hydroxybetulinic acid (also known as anemosapogenin) are extensively used as templates for the design of anticancer and antiviral compounds. In sharp contrast, acankoreagenin has been exploited only occasionally and its mechanism of action remains superficially understood. The compound is at the origin of many glycoside derivatives, the acankoreosides, but they have been equally little considered thus far. However, compounds like Ack-A and -B are efficient modulators of HMGB1 (26). Methods have been described to purify ACK from leaves of Acanthopanax gracilistylus (32,41) and there is good evidence for its anti-inflammatory potency in different models. There is no reason not to use further ACK as a scaffold for the design of bioactive molecules. Hopefully, this review will achieve its objective, to shed light on an almost forgotten natural molecule which could be useful to the design of modern therapeutic agent: a lesson learned from the TCM WuJiaPi, known to treat inflammatory conditions.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-21-1). CB serves as an unpaid editorial board member of Longhua Chinese Medicine from Jul 2020 to Jun 2022. The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brand E, Leon C, Nesbitt M, et al. Economic botany collections: A source of material evidence for exploring historical changes in Chinese medicinal materials. J Ethnopharmacol 2017;200:209-27. [Crossref] [PubMed]
- Han J, Pang X, Liao B, et al. An authenticity survey of herbal medicines from markets in China using DNA barcoding. Sci Rep 2016;6:18723. [Crossref] [PubMed]
- Ma L, Gao W, Chen F, et al. HS-SPME and SDE combined with GC-MS and GC-O for characterization of flavor compounds in Zhizhonghe Wujiapi medicinal liquor. Food Res Int 2020;137:109590 [Crossref] [PubMed]
- Kim MK, Jang GH, Yang DC, et al. Molecular Authentication of Acanthopanacis Cortex by Multiplex-PCR Analysis Tools. Korean J Plant Res 2014;27:680-6. [Crossref]
- Shoji J, Kawanishi S, Sakuma S, et al. Studies on the chemical constituents of Chinese drug "Wujiapi". Chem Pharm Bull (Tokyo) 1967;15:720-3. [Crossref] [PubMed]
- Sakuma S, Kawanishi S, Shoji J, et al. Constituents of Chinese crude drug "Wujiapi". I. Studies on the aglycones of steroidal glycosides of pei-wujiapi. (1). Chem Pharm Bull (Tokyo) 1968;16:326-31. [Crossref] [PubMed]
- Sakuma S, Kawanishi S, Shoji J. Constituents of the Chinese crude drug "Wujiapi". IX. Structure of glycoside H2 a potentiator of NGF-mediated nerve fiber outgrowth. Chem Pharm Bull (Tokyo) 1980;28:163-8. [Crossref] [PubMed]
- Song Y, Deng Y, Huang D, et al. LC-MS/MS determination and pharmacokinetic study of four lignan components in rat plasma after oral administration of Acanthopanax sessiliflorus extract. J Ethnopharmacol 2012;141:957-63. [Crossref] [PubMed]
- Chen S, Xu Y, Liang D, et al. The complete chloroplast genome of Eleutherococcus trifoliatus (Araliaceae): a wild edible plant in the coastal region of South China. Mitochondrial DNA B Resour 2020;5:513-4. [Crossref] [PubMed]
- Jia A, Zhang Y, Gao H, et al. A review of Acanthopanax senticosus (Rupr and Maxim.) harms: From ethnopharmacological use to modern application. J Ethnopharmacol 2021;268:113586 [Crossref] [PubMed]
- Załuski D, Kuźniewski R. In Vitro Anti-AChE, Anti-BuChE, and Antioxidant Activity of 12 Extracts of Eleutherococcus Species. Oxid Med Cell Longev 2016;2016:4135135 [Crossref] [PubMed]
- Adamczyk K, Olech M, Abramek J, et al. Eleutherococcus Species Cultivated in Europe: A New Source of Compounds with Antiacetylcholinesterase, Antihyaluronidase, Anti-DPPH, and Cytotoxic Activities. Oxid Med Cell Longev 2019;2019:8673521 [Crossref] [PubMed]
- Shan BE, Zeki K, Sugiura T, et al. Chinese medicinal herb, Acanthopanax gracilistylus, extract induces cell cycle arrest of human tumor cells in vitro. Jpn J Cancer Res 2000;91:383-9. [Crossref] [PubMed]
- Shan BE, Fu XM, Hua ZX, et al. Zhongguo Zhong Xi Yi Jie He Za Zhi 2005;25:825-8. [Study on mechanism of the anti-tumor activity of Acanthopanax gracilistylus]. [PubMed]
- Shan BE, Yoshita Y, Sugiura T, et al. Suppressive effect of Chinese medicinal herb, Acanthopanax gracilistylus, extract on human lymphocytes in vitro. Clin Exp Immunol 1999;118:41-8. [Crossref] [PubMed]
- Zhang Z, Dong J, Liu M, et al. Therapeutic Effects of Cortex acanthopanacis Aqueous Extract on Bone Metabolism of Ovariectomized Rats. Evid Based Complement Alternat Med 2012;2012:492627 [Crossref] [PubMed]
- Ahn S, Singh P, Jang M, et al. Gold nanoflowers synthesized using Acanthopanacis cortex extract inhibit inflammatory mediators in LPS-induced RAW264.7 macrophages via NF-kappaB and AP-1 pathways. Colloids Surf B Biointerfaces 2017;160:423-8. [PubMed]
- Xian LN, Qian SH, Li ZL. Zhong Yao Cai 2010;33:538-42. [Studies on the chemical constituents from the stems of Acanthopanax gracilistylus]. [PubMed]
- Xu HB, Yang TH, Xie P, et al. Cyperane-Type and Related (Nor)Sesquiterpenoids from the Root Bark of Acanthopanax gracilistylus and Their Inhibitory Effects on Nitric Oxide Production. J Nat Prod 2020;83:1453-60. [Crossref] [PubMed]
- Xu HB, Yang TH, Xie P, et al. LC-MS guided isolation of gracilistones A and B, a pair of diastereomeric sesquiterpenoids with an unusual tetrahydrofuran-fused tricyclic skeleton from Acanthopanax gracilistylus and their potential anti-inflammatory activities. Fitoterapia 2018;130:265-71. [Crossref] [PubMed]
- Yang J, Qian S, Cai X, et al. Chikusetsusaponin IVa Butyl Ester (CS-IVa-Be), a Novel IL6R Antagonist, Inhibits IL6/STAT3 Signaling Pathway and Induces Cancer Cell Apoptosis. Mol Cancer Ther 2016;15:1190-200. [Crossref] [PubMed]
- Liu XQ, Chang SY, Park SY, et al. A new lupane-triterpene glycoside from the leaves of Acanthopanax gracilistylus. Arch Pharm Res 2002;25:831-6. [Crossref] [PubMed]
- Yook CS, Liu XQ, Chang SY, et al. Lupane-triterpene glycosides from the leaves of Acanthopanax gracilistylus. Chem Pharm Bull (Tokyo) 2002;50:1383-5. [Crossref] [PubMed]
- Nhiem NX, Tung NH, Kiem PV, et al. Lupane triterpene glycosides from leave of Acanthopanax koreanum and their cytotoxic activity. Chem Pharm Bull (Tokyo) 2009;57:986-9. [Crossref] [PubMed]
- Zou QP, Liu XQ, Huang JJ, et al. Inhibitory effects of lupane type triterpenoid saponins from the leaves of Acanthopanax gracilistylus on lipopolysaccharide-induced TNF α, IL 1β and high mobility group box 1 release in macrophages. Mol Med Rep 2017;16:9149-56. [Crossref] [PubMed]
- Yang BY, Zhou YQ, Liu Y, et al. Ent-kaurane diterpenoids from the pericarps of Datura metel L. acted on the vascular endothelial cells via TRPC6 and NF-κB protein. Med Chem Res 2017;27:115-21. [Crossref]
- Xie XX, Jiang ZJ, Cheng ZH, et al. Preparative separation and quantitative determination of two kaurenoic acid isomers in root barks of Acanthopanax gracilistylus. Chin J Nat Med 2017;15:625-30. [Crossref] [PubMed]
- Huang L, Zhao H, Huang B, et al. Acanthopanax senticosus: review of botany, chemistry and pharmacology. Die Pharmazie-An International Journal of Pharmaceutical Sciences 2011;66:83-97. [PubMed]
- Lau KM, Yue GG, Chan YY, et al. A review on the immunomodulatory activity of Acanthopanax senticosus and its active components. Chin Med 2019;14:25. [Crossref] [PubMed]
- Liu XQ, Zheng LS, Weng LN, et al. Smashing Tissue Extraction of Active Triterpenoids of Anti-HMGB1 from Leaves of Acanthopanax gracilistylus W.W.Smith. J Central South Univ 2011;42:68-76. (Science and Technol).
- Dai L, Liu XQ, Xie X, et al. Characterization of stereostructure by X-ray and technology of extracting in combination hydrolysis in situ of acankoreanogenin from leaves of Acanthopanax gracilistylus W. W. Smith. J Cent South Univ 2014;21:3063-70. [Crossref]
- Sithisarn P, Jarikasem S, Thisayakor K. Acanthopanax trifoliatus, a Potential Adaptogenic Thai Vegetable for Health Supplement. Phytochemicals. In: Rasooli I. Bioactivities and Impact on Health. 2011. doi:
10.5772/27762 .10.5772/27762 - Adam G, Lischewski M, Phiet HV, et al. 3αHydroxy-Lup-20(29)-ene-23,28-dioic acid from Schefflera octophylla. Phytochemistry 1982;21:1385-7. [Crossref]
- Sung TV, Lavaud C, Porzel A, et al. Triterpenoids and their glycosides from the bark of Schefflera octophylla. Phytochemistry 1992;31:227-31. [Crossref] [PubMed]
- Chen Y, Tao S, Zeng F, et al. Antinociceptive and anti-inflammatory activities of Schefflera octophylla extracts. J Ethnopharmacol 2015;171:42-50. [Crossref] [PubMed]
- Liu X, Niu Y, Liu J, et al. Efficient Extraction of Anti-Inflammatory Active Ingredients from Schefflera octophylla Leaves Using Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with HPLC. Molecules 2019;24:2942. [Crossref] [PubMed]
- Zhang SR. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1990;12:198-202. [Effect of 3-hydroxy-lup-20(29)en-23-28-dioic acid on rat experimental gastric ulcers]. [PubMed]
- Li Y, Jiang R, Ooi LS, et al. Antiviral triterpenoids from the medicinal plant Schefflera heptaphylla. Phytother Res 2007;21:466-70. [Crossref] [PubMed]
- Liu XQ, Zou QP, Huang JJ, et al. Inhibitory effects of 3alpha-hydroxy-lup-20(29)-en-23, 28-dioic acid on lipopolysaccharide-induced TNF-alpha, IL-1beta, and the high mobility group box 1 release in macrophages. Biosci Biotechnol Biochem 2017;81:1305-13. [Crossref] [PubMed]
- Cao KY, Qiao CF, Zhao J, et al. Quantitative analysis of acankoreoside A and acankoreagenin in the leaves of Schefflera octophylla and Schefflera actinophylla using pressurized liquid extraction and high-performance liquid chromatography coupled with evaporative light scattering detection. J Sep Sci 2015;38:2201-7. [Crossref] [PubMed]
- Lu MX, Yang Y, Zou QP, et al. Anti-Diabetic Effects of Acankoreagenin from the Leaves of Acanthopanax gracilistylus Herb in RIN-m5F Cells via Suppression of NF-kappaB Activation. Molecules 2018;23:958. [Crossref] [PubMed]
- Zhang BX, Li N, Zhang ZP, et al. Protective effect of Acanthopanax gracilistylus-extracted Acankoreanogenin A on mice with fulminant hepatitis. Int Immunopharmacol 2011;11:1018-23. [Crossref] [PubMed]
- Xue J, Suarez JS, Minaai M, et al. HMGB1 as a therapeutic target in disease. J Cell Physiol 2021;236:3406-19. [Crossref] [PubMed]
- Chen M, Qin Y, Ma H, et al. Downregulating NF-κB signaling pathway with triterpenoids for attenuating inflammation: in vitro and in vivo studies. Food Funct 2019;10:5080-90. [Crossref] [PubMed]
- Jiang S, Zhang K, He Y, et al. Synergistic effects and mechanisms of impressic acid or acankoreanogein in combination with docetaxel on prostate cancer. RSC Adv 2018;8:2768-76. [Crossref]
- Jiang S, Li DL, Chen J, et al. Synergistic Anticancer Effect of Gemcitabine Combined With Impressic Acid or Acankoreanogein in Panc-1 Cells by Inhibiting NF-κB and Stat 3 Activation. Nat Prod Commun 2020;15:1-7. [Crossref]
- Li XJ, Zou QP, Wang X, et al. Lupane Triterpenes from the Leaves of Acanthopanax gracilistylus. Molecules 2018;23:87. [Crossref] [PubMed]
- Lim H, Min DS, Yun HE, et al. Impressic acid from Acanthopanax koreanum, possesses matrix metalloproteinase-13 down-regulating capacity and protects cartilage destruction. J Ethnopharmacol 2017;209:73-81. [Crossref] [PubMed]
- Cai XF, Lee IS, Shen G, et al. Triterpenoids from Acanthopanax koreanum root and their inhibitory activities on NFAT transcription. Arch Pharm Res 2004;27:825-8. [Crossref] [PubMed]
- Kim JA, Yang SY, Song SB, et al. Effects of impressic acid from Acanthopanax koreanum on NF-kappaB and PPARgamma activities. Arch Pharm Res 2011;34:1347-51. [Crossref] [PubMed]
- Jin SW, Pham HT, Choi JH, et al. Impressic Acid, a Lupane-Type Triterpenoid from Acanthopanax koreanum, Attenuates TNF-alpha-Induced Endothelial Dysfunction via Activation of eNOS/NO Pathway. Int J Mol Sci 2019;20:5772. [Crossref] [PubMed]
- Yook CS, Kim IH, Hahn DR, et al. A lupane-triterpene glycoside from leaves of two Acanthopanax. Phytochemistry 1998;49:839-43. [Crossref]
- Zhang JY, Pu SB, Qian SH, et al. Zhong Yao Cai 2011;34:226-9. [Studies on the chemical constituents in fruits of Acanthopanax gracilistylus]. [PubMed]
- Chang SY, Yook CS, Nohara T. Two new lupine-triterpene glycosides from leaves of Acanthopanax koreanum. Chem Pharm Bull 1998;46:163-5. [Crossref]
- Chang SY, Yook CS, Nohara T. Lupane-triterpene glycosides from leaves of Acanthopanax koreanum. Phytochemistry 1999;50:1369-74. [Crossref]
- Park SY, Choi HS, Yook CS, et al. A new lupane glycoside from the leaves of Acanthopanax koreanum. Chem Pharm Bull (Tokyo) 2005;53:97-99. [Crossref] [PubMed]
- Choi HS, Kim HJ, Nam SG, et al. Lupane glycosides from the leaves of Acanthopanax koreanum. Chem Pharm Bull (Tokyo) 2008;56:1613-1616. [Crossref] [PubMed]
- Nguyen XN, Phan VK, Chau VM, et al. Lupane-type triterpene glycosides from the leaves of Acanthopanax koreanum and their in vitro cytotoxicity. Planta Med 2010;76:189-194. [Crossref] [PubMed]
- Kim SC, Kang JI, Park DB, et al. Promotion effect of acankoreoside J, a lupane-triterpene in Acanthopanax koreanum, on hair growth. Arch Pharm Res 2012;35:1495-1503. [Crossref] [PubMed]
- Nhiem NX, Kiem PV, Minh CV, et al. Structure-activity relationship of lupane-triterpene glycosides from Acanthopanax koreanum on spleen lymphocyte IL-2 and IFN-gamma. BioOrg Med Chem Lett 2010;20:4927-4931. [Crossref] [PubMed]
- Dat LD, Thao NP, Luyen BT, et al. A new saponin from Acanthopanax koreanum with anti-inflammatory activity. Arch Pharm Res 2017;40:311-7. [Crossref] [PubMed]
- Luo Y, Huang J, Xu L, et al. Efficacy of Chinese herbal medicine for lumbar disc herniation: a systematic review of randomized controlled trials. J Tradit Chin Med 2013;33:721-726. [Crossref] [PubMed]
- Sonnenbora U, Hänsel R. Eleutherococcus Senticosus. In: De Smet PAGM, Keller K, Hänsel R, Chandler RF. editors. Adverse Effects of Herbal Drugs 2. Adverse Effects of Herbal Drugs, vol 2. Berlin, Heidelberg: Springer. 1993. doi:
10.1007/978-3-642-48906-8_12 .10.1007/978-3-642-48906-8_12
Cite this article as: Bailly C. The traditional Chinese medicine WuJiaPi (Acanthopanacis cortex) and its main anti-inflammatory terpenoids. Longhua Chin Med 2021;4:14.