
The regulation of Chinese medicine in Thailand
Introduction
There is historical evidence shows that some Chinese people came in contact with and lived in the place that is now known as Thailand even before the establishment of Sukhothai Kingdom, the first Kingdom of Thailand (1). During the Sukhothai period [1249–1438], the first relation with Yuan dynasty was initiated in the reign of King Ramkhamhaeng and Sukhothai began sending trade missions to China (2). The official relation and trade with China continued through Ayutthya [1350–1767] and Rattankosin (1782–present) periods along with continuous migration of Chinese people to live in Thailand since Ayutthaya period until the early 19th century. According to the data in 2012, among 50 million Chinese emigrants or oversea Chinese, 9.35 million came to live in Thailand (the biggest number in the world) accounting for about 14% of the population with 25 million partial Chinese ancestry (3).
Significant evidence of the role of traditional Chinese medicine (TCM) in Thailand can be dated back to the reign of King Narai [1656–1688], the most prosperous time of Ayutthaya period with trading and diplomatic activities with many countries, e.g. China, Japan, France, Portugal, and Persia. One of seven foreign doctors who served in the royal court was a Chinese doctor and his TCM herbal prescribed for the King was recorded in ‘Kampi Thad Phra Narai (palm leaf edition)’, the oldest accessible classical treatise of Thai traditional medicine (TTM) (4). During Ayutthaya and early Rattanakosin period, TTM doctors had learned about valuable Chinese materia medica from the practice of TCM doctors. This led to the adoption and use of several Chinese herbal materials as a part of herbal ingredients in Thai traditional medicine, e.g., baizhi, chuan xiong, da huang, di huang, guiwei, gancao, etc. (5). At the turn of the 20th century, the first TCM hospital (Tian Fah Foundation Hospital) and the first Chinese pharmacy were established in the China Town of Bangkok in 1903 and 1906, respectively, to mainly serve the Chinese community there, and later on in 1925 the Chinese Medicine Association of Thailand was founded (6).
An important step in recognizing the role of TCM in the health care of Thai people began when the Collaborating Center of Thai-Chinese Medicine was established as a division under the Department of Medicine, Ministry of Public Health in 1995 with the aim to improve the quality and standard of TCM care in Thailand to be an alternative form of health care for Thais. Under the leadership of the late Dr. Chawalit Santikitroongrueng, the first director of the Collaborating Center, two Memorandums of Understanding between the Ministry of Public Health of Thailand and the People’s Republic of China were signed in 1997 and 2000 and the first three-month training course on acupuncture for Thai medical doctors was started in 1998 in collaboration with Longhua Hospital, Shanghai University of Traditional Chinese Medicine. This acupuncture training has continued as an annual activity of the Department of Thai Traditional and Alternative Medicine (DTAM) until present. In addition, it also paved the way towards the issuance of Ministerial Regulation in 2000 to establish an official licensing system for the practitioners of TCM under the Practice of the Art of Healing Act B.E. 2542 [1999] (6).
In October 2002, the Collaborating Center of Thai-Chinese Medicine, along with the Institute of Thai Traditional Medicine and the Coordination Center of Alternative Medicine, were transferred to the administration of the Department for the Development of Thai Traditional and Alternative Medicine [currently Department of Thai Traditional and Alternative Medicine (DTAM)], one of the two new departments under the Ministry of Public Health established under the Bureaucratic Reform Act B.E. 2545 [2002]. The Collaborating Center was then a working group under the Division of Alternative Medicine and later in 2004 became the Southeast Asian Institute of Thai-Chinese Medicine, a division-level institute of DTAM and it is now the Institute of Thai-Chinese Medicine (ITCM) (6). Since its establishment, DTAM and ITCM, in collaboration with universities teaching TCM, Huachiew TCM clinic, hospitals providing acupuncture treatment, TCM experts, Traditional Chinese Medicine Doctor Association Thailand, Thai Acupuncture and Herbal Medical Association, as well as network of TCM universities in China and the National Administration of Traditional Chinese Medicine, have significantly contributed to the development of TCM in Thailand in various ways. This paper will review such accomplishments, past and current situation and the regulation of Chinese medicine in Thailand.
Regulation and reality of studies in acupuncture and Chinese medicine
After the establishment of DTAM in 2002, Dr. Vichai Chokevivat, the first Director-General of DTAM and Dr. Chawalit Santikitroongrueng made a joint effort to start a five-year undergraduate teaching of TCM at university level in Thailand. This led to the establishment of the Faculty of Chinese medicine at Huachiew Chalermprakiet University in 2004 and the second TCM school at the College of Alternative Medicine, Chandrakasem Rajabhat University in 2006. The qualification of educational institutions teaching TCM were certified by the Office of the Higher Education Commission and the graduates initially received a Bachelor of Science degree in Chinese medicine.
After TCM was legally recognized as a branch of the practice of the art of healing in 2009, the Profession Commission in the Branch of TCM issued two notifications in 2011, namely, (I) the criteria and assessment form for the accreditation of educational institutions that produce graduates with degree or degree-equivalent certificate in the branch of TCM, and (II) standard criteria for the accreditation of educational institutions that provide a degree program or degree-equivalent certificate program in the branch of TCM. As a result, since then the schools and faculties offering Bachelor’s degree program in TCM have to be accredited by both the Profession Commission in the Branch of TCM and the Office of the Higher Education Commission.
These two notifications were later repealed and a new Notification of the Profession Commission on “the accreditation criteria for educational institutions that produce graduates with degree or degree-equivalent certificate in the branch of TCM B.E. 2561” came into force in 2018. A TCM school needs to file a self-assessment form and application form for institution accreditation to the Director of Bureau of Sanatorium and Art of Healing, Department of Health Service Support. The profession sub-commission on the assessment of TCM educational institutions will visit and assess each school and the school that is accredited will receive the Accreditation Certificate that will be valid for 5 years and re-assessment is needed before the certificate can be renewed.
The standard criteria for the accreditation of TCM educational institutions stated in the 2018 Notification of the Profession Commission of the Branch of Traditional Chinese Medicine cover 12 aspects. The criteria include (I) the qualification of educational institution, (II) faculty member, (III) qualification of students, (IV) study curriculum and teaching plan, (V) readiness in educational management, (VI) student care system and advisory system, (VII) building, premise, tools and equipment for teaching and learning, (VIII) welfare, sport and creation facilities, (IX) library and information media, (X) administration and management, (XI) budget, and (XII) quality assessment of teaching and learning and institution assessment. Some important standard criteria are for example:
- Bachelor’s degree curriculum in TCM must be at least 5 years with total credits of at least 174. The study period of a student must not exceed 10 years;
- There must be at least 5 full-time lecturers of the TCM course and the ratios of lecturers to students for the lecture and for the practice must be 1:25 and 1:8, respectively throughout the 5-year course;
- Educational institution must teach Chinese language for at least one academic year (two regular semesters plus one summer semester) to prepare first year students for future TCM study. Students must pass Chinese Proficiency Test (Hanyu Shuiping Kaoshi, HSK) level 4 before studying TCM subjects, and pass level 5 before clinical clerkship;
- Of all 174 credits in 5-year curriculum, not less than 30 are general study, 35 are basic medical sciences, 73 professional (TCM) subjects including clinical clerkship of not less than 1,200 hours (7).
At present, according to the Notification of the Profession Commission in the Branch of TCM, in 2020, there are 9 universities in Thailand, and 31 universities from China that have already been accredited (8,9) as shown in Table 1 (8-18) and Table 2 (8,9), respectively. In 2018, there are 182 TCM graduates (April 2018) from universities in Thailand and the number tends to increase every year. It is estimated that currently there are about 200 TCM students graduated from universities in Thailand each year (8).

Full table

Full table
For a person who would like to obtain a Bachelor’s Degree in TCM from a university abroad, he or she is requested to submit the university curriculum to the Profession Commission for consideration and accreditation, at least 6 months in advance before going abroad to attend that university. Within 5 years before this Notification came into effect, anyone who graduated from TCM universities abroad that had not previously been accredited by the Profession Commission, has to submit the curriculum of the university, together with his or her academic record for the consideration by the Profession Commission on an individual basis for the accreditation of the university. In addition, it is required that the educational level of the educational institution abroad he or she attended had to be accredited by the national authority of that country.
Moreover, ITCM, DTAM also contributed to the TCM education by cooperating with our TCM networks and experts both in Thailand and in China to develop some TCM textbooks in Thai language that were later approved by the Profession Commission in the branch of TCM as guidelines for the study of TCM (19), such as Basic Traditional Chinese Medicine (20), Handbook on the Use of Thai-Chinese Materia Medica (21), Acupuncture & Moxibustion Volume 1-2 (22,23), and Commonly Used Chinese Prescriptions in Thailand Volume 1-3 (24-26), etc.
The other way to study TCM in Thailand, specifically in acupuncture and moxibustion, is by attending the “Three-month Acupuncture and Moxibustion Training course” in which only licensed medical doctors are qualified to enroll. As previously mentioned, the first training was initiated by the Collaborating Center of Thai-Chinese Medicine in 1998 and still continued every year after the center was transferred to DTAM until the 12th class in 2004 when the training became the responsibility of Praboromarajchanok Institute, Ministry of Public Health which provided training for the 13th class in 2005 to the 28th class in 2013. From 2014 onward, the training has been organized by the Institute of Thai-Chinese Medicine (ITCM), DTAM again from the 29th class in 2014 until present (8).
Currently, this three-month acupuncture training course is offered annually for interested doctors by two institutions, namely the ITCM and the Royal Thai Army Medical Department, Ministry of Defense which started its first year of training in 2006. The three-month acupuncture and moxibustion training at ITCM is a 390-hour course comprising 240 hours of lectures on basic principles of TCM, theory of acupuncture, and demonstration and 150 hours of clinical practice (27). The training of both institutions have so far been conducted in collaboration with TCM universities in China that sent TCM doctors or professors who are acupuncture specialists to be lecturers. Medical doctors who completed the training course will receive the certificate from the university in China that provided the training. As of 2020, a total of 1,979 medical doctors in Thailand completed the 3-month acupuncture and moxibustion training course—718 were trained by ITCM, 612 by Praboromarajchanok Institute, and 649 by the Royal Thai Army Medical Department (8).
Regulation and reality of the professional practice of acupuncture and Chinese medicine
Medical Act B.E. 2466 [1923] was the first law in Thailand to regulate the practice of the practitioners of the art of healing, the license to practice the art of traditional healing was also issued to TCM doctors then (28). In 1936, the Medical Act was repealed and the Act for the Control of the Practice of the Art of Healing B.E. 2479 [1936] was enforced in October 1937 (29); however, the practice of TCM was not clearly mentioned or covered by this Act, some TCM doctors therefore practiced without TCM practice license at that time. It was not until the Practice of the Art of Healing Act B.E. 2542 [1999] and the Ministerial Regulation on “the Permission of a Person to Practice the Art of Healing Based on the Knowledge of Traditional Chinese Medicine” issued in 2000 came into effect that the licensing system for TCM practitioners began (30,31). This Act states that Thai nationals or other nationals who have resided in Thailand for more than 3 years and who have completed a five-year curriculum of TCM education from academic institutions abroad and have received TCM practice license from the country he or she graduated from would be eligible for the issuance of the license to practice TCM after passing TCM knowledge test given by the Commission on the Practice of the Art of Healing. In addition, Thai nationals or other nationals who have resided in Thailand for more than 3 years and who have studied TCM from ancestors and practiced TCM for a long period of time can also register and apply for the license, provided that they have to pass a 144-hour TCM training course, offered by the responsible sub-commission of the Commission on the Practice of the Art of Healing Act, before being eligible to take the TCM knowledge test. As a result, at the end of 2001, there were 114 persons who had passed the knowledge test and were issued licenses to practice TCM—of these, 11 were graduates with Bachelor’s degree in TCM from China and 103 persons were trained in TCM by ancestors and passed the 144-hour TCM training course. The last TCM training course for the latter group was given in 2009, and in 2011, among 400 licensed TCM doctors—88 were graduates of TCM from universities in Thailand and abroad, and 312 were trained by ancestors. As of September 2019, the cumulative number of TCM doctors receiving TCM practice licenses was 1,469 (32).
In 2009, the Royal Decree prescribing TCM as a branch of the practice of the art of healing under Article 5[5] of the Practice of the Art of Healing Act B.E. 2542 [1999] was promulgated (33). As a result, the Profession Commission in the Branch of Traditional Chinese Medicine was established to be responsible for the accreditation of educational institutions that teach TCM in Thailand and abroad, the development of professional standards and technical aspects of TCM, the registration and arrangement of TCM knowledge test for the issuance of TCM practitioner license, and the legal and ethical aspects for the practitioners.
In February 2013, the 2009 Royal Decree prescribing TCM as a branch of the practice of the art of healing was repealed when the Practice of the Art of Healing Act (No. 4) B.E. 2556 (2013) came into effect (34). This law which is still effective today declared TCM as a branch of the practice of the art of healing, the definition of TCM, and the composition of the ProfessionCommission in the Branch of Traditional Chinese Medicine that consists of 4 ex officio committees, 3 deans of universities that teach TCM, 3 TCM experts appointed by the Minister of Public Health, and 10 committees elected from licensed TCM practitioners, and the director of the Bureau ofSanatorium and Art of Healing serving as a committee and secretary. The main responsibilities of the Professions Commission are mentioned as above and a sub-commission was appointed for each responsible area.
Each year the Profession Commission will issue a Notification on “Criteria, procedures, and conditions for the enrollment and knowledge test for the registration and licensing of practitioners of the art of healing in the branch of traditional Chinese medicine” (35). TCM graduates, whether they are Thai nationals or other nationals, who wish to take the knowledge test, must submit the application form along with the necessary documents within the given date. For applicants who are not Thai nationals, they must also present their TCM practitioner licenses in their own countries. The applicants will be tested on TCM knowledge, professional laws and clinical skills and skills in manual therapy, as well as interviewed on professional ethics and attitude. The license issued will be valid for 5 years, and the procedure and condition for a license renewal will soon be prescribed by the Profession Commission in the Branch of TCM (36,37). Licensed TCM practitioners are required by law to practice under the conditions set out in the regulation prescribed by the Minister of Public Health and are not allowed to advertise their practice and expertise.
Meanwhile, the medical doctors who finished the three-month training in acupuncture and moxibustion, do not need another license to practice acupuncture therapy for their patients, because acupuncture is one of therapies or treatment modalities that a licensed medical doctor is allowed to practice as stated in Article 4 of the Medical Profession Act B.E. 2525 [1982] (38).
Regulation and reality of Chinese phytotherapy
Previously, the production, import, sale, advertisement, and registration of traditional medicine products, whether Thai traditional medicine products, TCM products or herbal medicine products, were regulated by the Drug Act B.E. 2510 [1967] (39), while herbal dietary supplements were regulated by the Food Act B.E. 2522 [1979] (40), which made it illegal to make a health claim of the products as they were regarded as food. Therefore, it was obvious that a new law is necessary to specifically regulate all herbal products. As a result, the Herbal Product Act B.E. 2562 [2019] (41) was promulgated and has come into effect since 29 June 2019. All herbal products which include traditional medicines, herbal medicines, and herbal health supplements are now regulated and monitored under the Herbal Products Act.
According to the Drug Act B.E. 2510 [1967], “traditional medicines” means drugs intended for use in the traditional practice of the art of healing or the treatment of animal diseases, or drugs that the Minister of Public Health notified as traditional drugs, or drugs that are licensed to register as traditional medicines, which consist of traditional household medicine and non-traditional household medicine (39).
According to the Herbal Products Act B.E. 2562 [2019], herbal products means:
- Herbal drugs and shall also include Thai traditional drugs, developed herbal drugs, traditional drugs for use in humans under the law on drugs, or drugs that are derived from the knowledge of alternative medicine as prescribed and notified by the Minister, for the treatment, cure and relief of human illnesses or the prevention of diseases;
- Products made from herbs or products of which active ingredients are herbs or derived from herbs that are ready for use in humans to improve their health or the function of their body, strengthen the structure or the function of human body or reduce the risk factors of a disease;
- Substances intended for use as ingredients in the production of herbal products;
- Other substances prescribed and notified by the Minister upon the recommendation of the Committee on Herbal Products as herbal products (41).
According to the Herbal Product Act, a herbal product will undergo a herbal product review process before it can be produced or imported by one of the three processes, depending on the risk of the product, namely listing, notification, or registration (41), while under the previous Drug Act only registration process was available. The new review processes by listing and notification are developed to shorten the time for the review of low-risk type of products and the time to launch herbal products to the market. The criteria and procedures for product listing, notification and registration are in process of drafting of the secondary laws to fully enforce the Act, in the meantime, all traditional medicines are reviewed by a registration process under the Drug Act.
TCM products registered in Thailand can be divided into two types, namely those that are locally manufactured and those that are imported from abroad which both have been registered as ‘traditional drugs’ according to the Drug Act B.E. 2510 [1967]. Registered TCM products must be sold in licensed pharmacies or licensed traditional pharmacies only, so that pharmacists or TCM doctor (if available) can give advice to the patient on how to take TCM medications.
A manufacturer or importer who intends to have a TCM product registered in Thailand must first file an application form for obtaining permission to produce or import a sample product for registration. After permission is granted, the applicant can then produce or import a sample product and send it to a certified laboratory for microbial analysis to comply with the microbial limits specified by Thai Herbal Pharmacopoeia (42). When the test result is obtained, the manufacturer or importer will then submit the application form for product registration together with the sample product, the microbial test result and other required documents to Thai Food and Drug Administration (TFDA). DTAM, in collaboration with TFDA, will then review and evaluate the submitted documents and the product for safety, efficacy and quality before the TCM product will be registered and the certificate of herbal product registration will be issued to the manufacturer or importer.
The review process for the registration of either Thai traditional medicine or TCM by DTAM is conducted by a committee of experts in the fields of Thai traditional medicine, Chinese medicine, and pharmaceutical sciences, as well as experienced officials from relevant government agencies, e.g., DTAM, TFDA and the Department of Medical Sciences. For a Chinese medicinal product, the committee will determine if the product formula is in accordance with the principles of Chinese medicine formulation to ensure its efficacy and safety and if the quality of the product is as specified. Sometimes the manufacturer or importer will need to submit more information or answer some questions that the committee may have on the product. The final decision of the committee will be sent to TFDA for further action whether to register or not.
On the consumer protection side, in contrast to the TCM situation in China, licensed pharmacies or traditional pharmacies in Thailand, where TCM products are sold, hardly have TCM practitioners as persons on duty and most Thai people still do not understand nor have enough knowledge about TCM. The approved indications of the TCM products must therefore be limited to the treatment of diseases or the relief of symptoms that Thai people can understand well and can be used for self-care, but not for the diseases or symptoms that require the diagnosis or treatment by TCM practitioners. In addition, necessary information required for the safe use of the product, e.g. contraindications, warnings and precaution, has to be provided in the package insert. However, in the future, if some TCM products are allowed by TFDA to be sold directly to TCM practitioners or to the hospitals providing TCM service, broader TCM-related indications can then be made on the label or package insert.
Level of acceptance by the population
According to Article 3 of the National Health Security Act, B.E. 2545 [2002], which is the primary legislation of the Universal Health Coverage (UHC) System that covers the largest number of beneficiaries (about 75% of Thais), Thai traditional medicine and alternative medicine (including TCM) services recognized by the law on the practice of the art of healing are included in the “health services” that will be covered by the UHC (43). In public health service facilities under the Office of the Permanent Secretary, Ministry of Public Health, the integration of Thai traditional medicine service, our own traditional wisdom of healthcare, in the health care system has higher priority than the TCM service. Currently, most public hospitals, where there are medical doctors trained in acupuncture or TCM practitioners, provide only acupuncture services. From the latest survey by the Institute of Thai-Chinese Medicine, as of April 2020, there are a total of 272 hospitals, both public and private, that provide acupuncture services all over the country. Of these, 224 are hospitals in the 12 health service regions of the Ministry of Public Health, and 48 are hospitals under other ministries, Bangkok Metropolitan Administration, universities, or private hospitals. In contrast, in Thailand, almost all full service of TCM are currently provided in private clinics. As of September 2019, across the country, there are 259 private TCM clinics (107 in Bangkok and 152 in other regions) that have been registered with the Bureau of Sanatorium and Art of Healing, Department of Health Service Support, Ministry of Public Health (32).
However, until fiscal year 2020, the acupuncture service has not yet been covered by UHC or by the Social Security Scheme (SSS) which is the health security system for people who work in the private sector, while the acupuncture service is covered by the Civil Servant Medical Benefit Schemes (CSMBS) for government officials, their parents and children under the age of 20. Other practices, if available, such as moxibustion, prescription of TCM medicines, tuina, cupping, are not covered by any of the 3 health security systems.
During fiscal year 2020, DTAM has collaborated with the National Health Security Office (NHSO), the government agency in charge of UHC, to increase UHC beneficiaries’ access to acupuncture as another treatment modality, in addition to physiotherapy, in the service package for the treatment of stroke patients in intermediate care (IMC) in hospitals that have medical doctors trained in acupuncture or TCM practitioners to provide the service. Beginning with fiscal year 2021, UHC will allocate an additional per capita budget for hospitals that provide acupuncture or electro-acupuncture therapy for UHC beneficiaries who just have had a stroke for rehabilitation during IMC. The additional per capita budget will be paid for each session of electro-acupuncture for a total of 20 sessions within 6 months after stroke, along with an additional per capita budget after completion of treatment, if the hospital can fully record Barthel index assessment scores for all 20 visits. This is the first time that NHSO adds an alternative medicine therapy in the service package. If the results of the clinical trial to be conducted in 2021 to assess the efficacy and safety of acupuncture plus physiotherapy compared with physiotherapy alone in stroke patients turn out well as expected, the NHSO is expected to expand the acupuncture service package to cover other diseases or symptoms in the future.
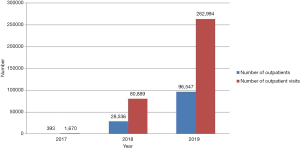
The acceptance of TCM by Thai people is increasing as seen from the number of TCM practitioners working in public health service facilities which increased from 48 in 2015 to 179 in 2020, along with the growing number of outpatients receiving TCM services in public health service facilities as shown in Figure 1 (44).
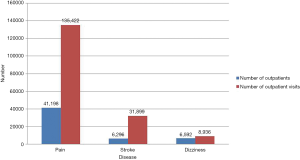
As seen in Figure 2 (44), pain, stroke and dizziness were three major health problems that cause patients to seek TCM or acupuncture treatment in public health service facilities.
The increase in acceptance of TCM is most evident in TCM private clinics where full TCM services are provided, as seen in the growth of Huachiew TCM Clinic, the largest TCM clinic in Thailand located in Bangkok, which has provided TCM service for outpatients only since its establishment in 1995. At the beginning there were only 7 full-time TCM practitioners, and over the years to the present, Huachiew TCM Clinic has expanded their facilities and increased the number of TCM practitioners to 42 to accommodate the growing number of patients. To expand complete TCM services to people in other regions of the country, the first branch of Huachiew TCM Clinic was established in 2014 in Nakonratchasima province in the northeast of Thailand and in 2019 the second branch in Sriracha, Chonburi province in the east, with 7 TCM practitioners in each branch. For over 25 years providing and leading TCM services in Thailand, on 15 December 2018, Huachiew TCM Clinic was selected by the National Administration of Traditional Chinese Medicine of the People’s Republic of China, as the “China-Thailand Traditional Chinese Medicine Center” in the area of TCM service in Thailand (45).
Conclusions
In addition to traditional Thai medicine, Chinese medicine is another of the two legally recognized systems of traditional medicine in Thailand. Although the role of Chinese medicine in the public health service system is second to traditional Thai medicine, the recognition and acceptance of Chinese medicine by healthcare professionals and the public has gradually increased in the past 20 years, along with the increase in the number of licensed TCM practitioners each year. In order to ensure the level of TCM graduates, the quality of practice of TCM doctors, and the quality of TCM products, various regulatory measures and laws have been developed, enforced, amended and changed over time.
Acknowledgments
The authors would like to thank Mrs. Boonjai Limsila, Director of the Institute of Thai-Chinese Medicine, for providing TCM information as an input for the preparation of this review article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ramon Calduch Farnòs) for the series “Regulation of Chinese Medicine in the Different Countries of the World” published in Longhua Chinese Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-20-36). The series “Regulation of Chinese Medicine in the Different Countries of the World” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wongthes S. Suvarnabhumi in ASEAN: China - Thailand have a common culture for thousands of years before the birth of Sukhothai [Internet]. Thailand: Matichon Online; 2020 Jan 2 [cited 2020 Aug 26]. Available online: https://www.matichon.co.th/prachachuen/prachachuen-scoop/news_1860131
- World Heritage Encyclopedia. Sukhothai Kingdom [Internet]. United States: Project Gutenberg Self-Publishing Press; [cited 2021 Mar 25]. Available online: http://self.gutenberg.org/articles/eng/Sukhothai_Kingdom
- Chinese Diaspora [Internet]. Berlin: Academy for Cultural Diplomacy; [cited 2021 Mar 25]. Available online: https://www.culturaldiplomacy.org/academy/index.php?chinese-diaspora
- Picheansoonthon C, Maenmas C, Jirawong V. Description of King Narai’s Medicines Treatise. Bangkok: Amarin Printing and Publishing Public Co., Ltd, 2001.
- Monographs of Selected Thai Materia Medica Volume 1. Bangkok: Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, 2009.
- Thai Traditional and Alternative Medicine Health Profile. Thai Traditional Medicine, Indigenous Medicine and Alternative Medicine 2011-2013. Bangkok: Office of Information and Evaluation, Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health; Chapter 8, One Decade of Traditional Chinese Medicine in Thailand; 2014:345-406.
- Notification of Board of TCM Practice of Healing Arts, Re: Approval Criteria for educational institutions that provide TCM degree or certificate equivalent to TCM degree, B.E. 2561. (2018). Available online: https://hss.moph.go.th/fileupload_doc/2018-07-04-1-18-44971839.PDF
- TCM Database [Internet]. Nonthaburi: Institute of Thai-Chinese Medicine, Department of Thai Traditional and Alternative Medicine, Ministry of Public Health; [cited 2020 Sep 29]. Available online: https://tcm.dtam.moph.go.th/
- Notification of Board of TCM Practice of Healing Arts, Re: Approval of educational institutions offering TCM courses from abroad, B.E. 2553 (No.2). (2010).
- History [Internet]. Samutprakan: Faculty of Traditional Chinese Medicine, Huachiew Chalermprakiet University; [cited 2020 Aug 26]. Available online: http://cmed.hcu.ac.th/index.php
- History [Internet]. Bangkok: College of Alternative Medicine, Chandrakasem Rajabhat University; [cited 2020 Aug 26]. Available online: https://amc.chandra.ac.th/
- History [Internet]. Nakhonratchasima: Faculty of Traditional Chinese Medicine, Nakhonratchasima College; [cited 2020 Aug 26]. Available online: http://tcm.nmc.ac.th/th/home.php
- History [Internet]. Chiang Rai: Faculty of Oriental Medicine, Chiang Rai Collage; [cited 2020 Aug 26]. Available online: http://www.crc.ac.th/th/faculty/oriental-medicine/about
- School of Integrative Medicine [Internet]. Chiang Rai: School of Integrative Medicine, Mae Fah Luang University; [cited 2020 Aug 26]. Available online: https://www.mfu.ac.th/education/school/sch-integrative-medicine.html
- History [Internet]. Pathumthani: College of Oriental Medicine, Rangsit University; [cited 2020 Aug 26]. Available online: https://www.rsu.ac.th/orientalmed/
- Faculty of Medicine [Internet]. Payao: Faculty of Medicine, University of Payao; [cited 2020 Aug 26]. Available online: http://www.medicine.up.ac.th/
- Academic partners [Internet]. Pathumthani: Chulabhorn International College of Medicine, Thammasat University; [cited 2020 Aug 26]. Available online: http://www.cicm.tu.ac.th/cicmN4/index.php
- TCM [Internet]. Samut Songkhram: College of Allied Health Sciences, Suan Sunandha Rajabhat University; [cited 2020 Aug 26]. Available online: http://tcm.ahs.ssru.ac.th/th
- Notification of Profession Commission in the Branch of Traditional Chinese Medicine, Re: Textbooks used as guidelines for a person who intends to practice in traditional Chinese medicine, B.E. 2554. (2011).
- Basic Traditional Chinese Medicine. Bangkok: Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, 2008.
- Handbook on the Use of Thai-Chinese Materia Medica. Bangkok: Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, 2008.
- Acupuncture & Moxibustion Volume 1. Bangkok: Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, 2008.
- Acupuncture & Moxibustion Volume 2. Nonthaburi: Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, 2010.
- Commonly Used Chinese Prescriptions in Thailand Volume 1. Bangkok: Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, 2006.
- Commonly Used Chinese Prescriptions in Thailand Volume 2. Bangkok: Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, 2008.
- Commonly Used Chinese Prescriptions in Thailand Volume 3. Bangkok: Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health, 2010.
- Theansuwan W. Orientation [unpublished lecture]. 35th Acupuncture Training Course (3-month) for Medical Doctors in the Year 2020, Institute of Thai-Chinese Medicine, Department of Thai Traditional and Alternative Medicine, Ministry of Public Health; lecture given – 2020 Mar 2.
- Medical Act B.E. 2466. (1930). Available online: https://dl.parliament.go.th/handle/lirt/16428
- Act for the Control of the Practice of the Art of Healing B.E. 2479. (1936). Available online: https://dl.parliament.go.th/handle/lirt/16913
- Practice of the Art of Healing Act B.E. 2542 (1999). Available online: http://203.155.220.230/bmainfo/law/011/artdisease.pdf
- Ministerial Notification No.1 (B.E. 2543) (2000) on the Permission of a Person to Practice the Art of Healing Based on the Knowledge of Traditional Chinese Medicine. Available online: http://law.longdo.com/law/97/sub12244
- Public Health Calendar B.E. 2563 (2020). Bangkok: National Health Association of Thailand, 2020.
- Royal Decree B.E. 2552 Prescribing the Branch of Traditional Chinese Medicine as a Branch of the Practice of the Art of Healing under the Practice of the Art of Healing Act B.E. 2542. Available online: https://dl.parliament.go.th/handle/lirt/25970
- Practice of the Art of Healing Act (No.4) B.E. 2556. (2013). Available online: https://hss.moph.go.th/fileupload_doc_slider/2016-11-14-25-16-192407.pdf
- Notification of the Profession Commission in the Branch of Traditional Chinese Medicine on “Criteria, Procedures, and Conditions for the enrollment and Knowledge Test for the Registration and Licensing of Practitioners of the Art of Healing in the Branch of Traditional Chinese Medicine for the Year 2563 (2020)”. Available online: https://mrd-hss.moph.go.th/mrd1_hss/wp-content/uploads/2020/07/ประกาศรับสมัครสอบแพทย์จีน-63.pdf
- Ministerial Regulation on the Application for Registration and License, the Issuance of License, the Application for License Substitute and the Issuance of License Substitute for the Practice of the Art of Healing (No. 2) B.E. 2562 (2019). Available online: http://www.ratchakitcha.soc.go.th/DATA/PDF/2562/A/037/T_0001.PDF
- Invitation on Public Hearing of Continuing Education Criteria [Internet]. Nonthaburi: Bureau of Sanatorium and Art of Healing, Department of Health Service Support: 2020 Jun 19 [cited 2020 Aug 26]. Available online: https://mrd-hss.moph.go.th/mrd1_hss/?p=2368
- Medical Profession Act B.E. 2525. (1982). Available online: https://www.tmc.or.th/download/law-medical_2525.pdf
- Drug Act B.E. 2510. (1967). Available online: http://203.155.220.230/bmainfo/law/011/medicine2510.pdf
- Food Act B.E. 2522. (1979). Available online: https://www4.fisheries.go.th/local/file_document/20170227141540_file.pdf
- Herbal Product Act, B.E. 2562. (2019). Available online: http://www.fda.moph.go.th/Herbal/SitePages/law_herbal2.html
- Thai Herbal Pharmacopoeia 2019 Volume I and II. Bangkok: Department of Medical Sciences, Ministry of Public Health. 2019.
- National Health Security Act B.E. 2545. (2002). Available online: http://nih.dmsc.moph.go.th/law/pdf/031.pdf
- Standard report group on TCM services [Internet]. Health Data Center (HDC), Ministry of Public Health. [cited 2020 Aug 26]. Available online: https://hdcservice.moph.go.th/hdc/reports/page.php?cat_id=e67da2428ef09faaa68d7e92d1becb51
- History of Huachiew TCM Hospital [Internet]. Bangkok. Huachiew TCM Clinic; 2014 Oct 1 [cited 2020 Aug 26]. Available online: https://www.huachiewtcm.com/en/content/7416/history-of-huachiew-tcm-hospital
Cite this article as: Donnapee S, Wongpim K, Chuthaputti A. The regulation of Chinese medicine in Thailand. Longhua Chin Med 2021;4:3.

