
A narrative review of botanical characteristics, phytochemistry and pharmacology of Valeriana jatamansi jones
Introduction
The plant of Valeriana jatamansi Jones (family Caprifoliaceae), a perennial medicinal herb, is endemic to provinces of Yunnan, Sichuan, Guizhou and Shanxi in China and Himalaya of India, and it is also found in Ghutan, Pakistan, Nepal, Myanmar and North America. It is well known as “Zhizhu Xiang” and “Mati Xiang” in China, “Tagar” and “Indian valerian” in India (1,2). Due to the characteristics of being dioecious, polygamous or polygamomonoecious, the species reproduces sexually or asexually (3,4). As a member of Caprifoliaceae family, Valeriana jatamansi Jones has a long history of application in Europe and has been extensively used as medicine and spice. Therefore, medicinal application of this herb is now officially listed in the Chinese, Indian, British and European Pharmacopoeia. The roots/rhizomes of this plant have been widely utilized as a conventional medicine treating various diseases such as anxiety disorder, tranquilizing hypnotic, irritable bowel syndrome (IBS), epilepsy, snake poisoning and hyperlipidemia (4-10). As a main raw ingredient of the health product Tagara, this herb is a major component for the treatment of depressive insomnia clinically (11). It is also an important material of clinical experiential prescription Antianxietic Compound Prescription Capsule (ACPC) in China, composed of Zhizhu Xiang, Albizia cortex, Ziziphi Spinosae Semen Tostum and Junci Medulla (12). Apart from ACPC, Compound Mati Xiang Keli (composed of Mati Xiang, Atractylodes Lancea (Thunb.) DC, Asarum sieboldii Miq., Artemisia argyi, Amomum kravanh Pierre ex Gagnep., Euryale ferox Salisb. ex DC, Alisma plantago-aquatica Linn. and garden ginger, Ziziphus jujuba Mill.) and Valeriana jatamansi Jones decoction have been applied for the treatment of pediatric rotavirus enteritis (PRE) and can help to improve clinical efficacy through reducing the level of inflammatory indicators in infants (13-15). Of note, unlimited removal of this natural resources from the wild mainly for trade values resulted in a rapid reduction of the plants from the natural environment and thus was listed into the “endangered” category in India and Pakistan (16,17). And it would be soon to get noticed that the herb will be not in sustainable harvesting to satisfy demand. Therefore, in vitro propagation for large scale reproduction has been a replaceable method for commercial multiplication as well as conservation (16).
Among the complex chemical compositions of the plant, valepotriates and flavonoids are the major bioactive chemical constituents of roots/rhizomes. In addition, flavonoid, essential oil composition, sesquiterpenoids, lignin and polyphenols are also isolated from the species, which possess different bioactivities (10,18-22). Thus far, despite the popular clinical use of Valeriana jatamansi Jones, scientific evidences are not clarified to identify the effective chemical constituents responsible for the multiple biological activities. The quality control of the Zhizhu Xiang crude drugs is still to be improved and the medicinal potential of the isolated compounds from this herb has not been fully demonstrated yet. A narrative review of Valeriana jatamansi Jones could be useful for researchers, policymakers and manufacturers to achieve better conservation, management and obtain a holistic view of this important herbaceous plant.
As an important herbal medicine with great potential to be explored, another work such as the exact mechanism of how the bioactive compounds exert therapeutic effect on specific diseases and proper application of polyherbal combination should be studied further. Herein, this review mainly focuses on the elaborate analysis of Valeriana jatamansi Jones regarding botanical characteristics, phytochemistry, and pharmacology.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/lcm-20-54).
Materials and methods
The literature of Valeriana jatamansi Jones published from 1979 to March 2020 were collected from databases including PubMed, Elsevier, Web of Science, and China Network Knowledge Infrastructure, Google Scholar and Baidu Scholar. Valeriana jatamansi Jones, Valerianae Rhizoma, Zhizhu Xiang, Mati Xiang, Valeriana wallichii DC., Tagar, Mushkbala and Indian valerian were used as the key words.
Botanical characteristics
Valeriana jatamansi Jones is a perennial herb, 20–70 cm high, with specific aroma and mostly fibrous roots. Stems are usually branched and densely pubescent. The rhizomes are transverse, thick and lumpy, with dense internodes, and the petiole residues are tan. Its basal leaves are developed, the leaves are round to ovate, 2–10 cm long and 1.5–8 cm wide, the apex is short, sharp or dull, and the base is heart-shaped, the edge is microwave-shaped or has sparse denticles with short fluff, dark green on the top, light green on the bottom, all pubescent, 5–9 basal veins (23). Cauline leaves are undeveloped, with 2–3 pairs per stem and the lower part being heart-shaped round, nearly sessile, and the upper part being evergreen crack and sessile. Inflorescences are terminal cymes, bracts and bracteoles are rhombus-shaped. The corollas, 6 mm in length, are white and slightly reddish. There are 3 stamens inserted in the middle of corolla tube, pistils are protruding out of corolla with 3-lobed columnar, and the ovary is inferior. Bisexual flowers are larger, with 3–4 mm long (24,25). What’s more, the pistil and stamens are protruding from the corolla with equal length. Achenes are long-ovate, around 2 mm long, 1 mm wide, with both sides having pubescence. The flowering period occurs from May to July and the fruit are harvested from June to September (26). It is a shade loving herb and often cultivated in the humid and fertile soil, such as the grassland, forest, shrub, gully grassland, stream side, etc. and it can also be found on the top of the mountain below 3,000 m above sea level (27,28).
Bioactive compounds
Crude plant of Valeriana jatamansi Jones contains flavonoids (29,30), valepotriates (2,31,32), flavone glycosides (21), essential oils (22,33,34), lignans (35), phenolic compounds (20,36,37), sesquiterpenoids (38,39), bakkenollide type sesquiterpenoids (40), sesquiterpenoid glycosides (41) and other phytochemicals. Among them, valepotriates and flavonoids are the major compounds mainly isolated from the roots/rhizomes. The bioactivities of the major bioactive compounds and extracts of the herb are summarized in Table 1 and Table 2, respectively. They possess a broad spectrum of biochemical properties such as anti-oxidant (45,94), anti-inflammatory (86,90), neuroprotective (50,88), anti-virus (14,84), antidepressant (78,115), anti-tumor (51,52), gastrointestinal (7,116,117) and other activities. A great attention was paid to scientifically demonstrate links between the contents and the extracts of this herb. Among them, valtrate (8,45-47,54,67), acevaltrate (45-48), baldrinal (45,49), didrovaltrate (52,53) and 11-ethoxyviburtinal (7,44) are the most potent ingredients in treatment of various diseases like IBS, antispasmodic, analgesic, etc. As for another component, 6-methylapigenin and hesperidin (flavonoids) (30,118), as well as valeranone (sesquiterpenoids) (54) could predominantly affect the brain including regulating GABAA receptor and CNS. However, the chemical composition of volatile oil varies on the basis of the natural habitat as well as the extract methods used (119). It should be noteworthy that patchouli alcohol was the superior chemotype of the essential oil among three different countries in Europe (120). The results are in concordance with previous reports indicating that patchouli alcohol and maaliol were the top two superior chemotypes with the evidence of the plant grown in India (20,22,96,121). Conversely, plant material originated from the southwestern China was indicated that terpenoids are the rich constituents of the essential oil (122). Current research on patchouli alcohol chemotype essential oil demonstrated its remarkable functions of antioxidant properties in vitro and analgesic action in vivo (20,99). However, the exact mechanisms of chemical compounds mentioned above are still unknown due to the preliminary bioactivities in vitro.

Full table
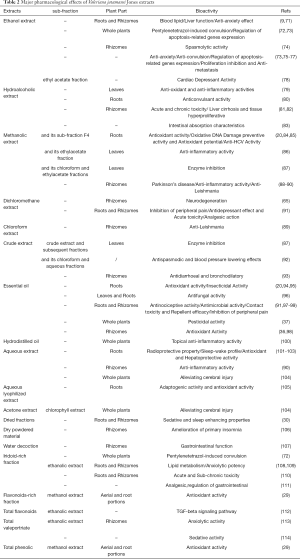
Full table
Pharmacological effects
The roots/rhizomes of this herbaceous plant have been used in conventional medicine as an alternative drug for a long history. In traditional medicine, Valeriana jatamansi Jones is widely utilized to treat depression, neurosis, sciatica, hysteria and is potential to be treated as an alternative to tranquilizer (92,123). A great number of literature has been reported on the pharmacological effects of different extracts of this herb, including water, ethanol and dichloromethane extract, and the major bioactive compounds such as valerian, 11-ethoxyviburtinal, IVHD-valtrate and hesperidin. The results of different research could be challenging to be summarized or compared with each other regarding the complexity and diversity of variant experimental models conducted.
Neuroprotective activities
Four bakkenolide-type sesquiterpenes and jatairidoids A-C (acylated iridoids), derived from the roots of Valeriana jatamansi Jones, could exert great neuroprotective effects through inhibiting the dopaminergic neuroblastoma SH-SY5Y neuronal cell death over 77.2%, compared with the positive control guanosine (less than 60%) (40,57). Another study reported compounds isolated from the roots and rhizomes including isopatrinioside, (4b,8b)-8-methoxy-3-methoxy-10-methylene-2,9-dioxatricyclo[4.3.1.03,7]decan-4-ol, vibutinal, (1S,3R,5R,7S,8R,9S)-3,8-epoxy-1-O-ethyl-5-hydroxyvalech lorine exhibited apparent neuroprotective effects on the neuronal death of rat pheochromocytoma (PC12) cells induced by CoCl2 (41,42,50). Moreover, the results of administration of the rhizomes extract and its isolate valeric acid revealed that both of the administration could decrease lipid peroxidation and reduce GSH in brains compared to normal rats. It is noteworthy that the effect of improving memory with the administration of valeric acid (40 mg/kg) was significant compared with the rhizomes extract in morris water maze test and elevated plus maze test. The results might validate the fact of neuroprotective potentiality of the herb and its component valeric acid in ameliorating memory cognition and retention in model dementia rats (65). Similarly, the aqueous extract of the whole plant was shown to moderately inhibit cerebral injury in global cerebral ischaemia mice with 14.9% cerebral infarct size as compared to control group mice (34.2%), suggesting its role playing in neuroprotective effect (104). What’s more, another study on orally administration of Valeriana jatamansi Jones rhizome extract (VRE) in MPTP-induced Parkinson’s disease (PD) mice was performed. Based on the results of alteration of striatal dopamine, TH protein and tyrosine hydroxylase levels, amelioration of LPO and ROS, improvement of antioxidants levels in PD mice, it was effectively demonstrated that neuroprotective effect of VRE might be possibly achieved through its ability to improve the antioxidant activities and to attenuate neuroinflammation (88). As a potential analgesic agent, the attention of N-type and T-type calcium channels was brought into consideration. It was firstly found that isolated compounds valeriananoid A, velerivaltrate A, didrovaltratisovaleroyloxyhydrin, valtrate and acevaltrate could not only exhibit antagonistic activities with EC50 values of 7.8, 4.33, 2.70, 2.18 and 1.13 µM, respectively, but could also act as Cav2.2 (N type) and Cav3.1 (T type) dual-calcium-channel-inhibitor with rough average inhibitory ratio values 60.74% and 36.34%, respectively (47). Later, it was further investigated that ethyl acetate-soluble part of ethanol extract could exhibit the most potent suppression of Cav2.2 peak current (20 mV) comparing with H2O and n-BuOH soluble parts. Based on bioassay-guided search, the active constituents Jatamanvaltrate T and valtrate hydrin B8, responsible for Cav2.2 N-type voltage-gated calcium channels (VGCCs) inhibitor, were identified to play significant roles as allosteric modulators in mitigating nociception (62).
The isolates from the roots of the plant including clovane-2β-isovaleroxy-9α-ol, jatamanvaltrates R-Q, valeriananoid A-E, volvaltrate B, valeriotetrate A, valeriotetrate B, 8, 11-desoidodidrovaltrate, rupesin E and (3S, 4R, 5S, 7S, 8S, 9S)-3, 8-ethoxy-7-hydroxy-4, 8-dimethylperhydrocyclopenta[c] pyran (50 µM) were shown to exhibit acetylcholinesterase (AChE) activity suppression ratios less than 10%, indicating that compounds mentioned above might be not the active component on the inhibitory effects of AChE (38). Chloroform and ethyl acetate fractions of Valeriana jatamansi Jones leaves were active against AChE and butyrylcholinesterase (BChE) enzymes with IC50 values 61 and 58 µg/mL, respectively (87). Likewise, primary AChE inhibition assay on the essential oil was implied that only cis-ocimene showed strong AChE suppression activity (87.5%), followed with benzaldehyde (20.2%), acetophenone (19.5%), styrene (19.4%), as well as benzylalcohol (11.3%) (124).
Antidepressant and antianxiety activities
The antidepressant activity of Valeriana jatamansi Jones is mainly attributed to the bioactive compounds iridoids, flavonoid, valepotriates and essential oil composition. The ethanol extract and total valeportriate from roots and rhizomes were revealed to play a significant role in antianxiety activity and sedation and hypnogenesis. And after the animals being orally administrated with valtrate (for 10 d), serum corticosterone (CS) and the brain hippocampal tissue in the neurotransmitter 5-serotonin (5-HT), dopamine (DA) and norepinephrine (NE) levels were down-regulated, suggesting that the extract and total valeportriate from the herb could play a role in regulation of anxiety through thalamus-pituitary-adrenal (HPA) axis system and are presumed to be potential anxiolytic drug (8,113). A similar research on the ethanol extract also showed a significant reduction of 5-HT, NE and DA level in Sprague Dawley (SD) rats (77). Administration of SD rats with 0.3, 0.6 and 0.9 g/kg everyday of 95% ethanol extract i.p. significantly decreased the percentage of entrance into the open arm (OE%) and remaining in the open arm (OT%), as compared with the group injected with estazolam. At the meantime, five apoptosis-related genes expression were detected, the results implied that the apoptosis-related genes Ets-1, Elk-1, Bax, Apaf-1 and Bcl-2 expression were inconsistently expressed between estazolam-induced anxiety model rats and rats treated by the extract of the plant. Due to the up-regulation of five genes expression, it was inferred that the ethanol extract could be closely associated with the adjustment to a multitude of different cancers under stress (73). To further study the dysfunction of HPA in anxiety model rats, Yan et, al. found that blood β-endorphin (β-EP) and CS levels were higher in the anxiety model group rats than in the normal group, and the expression of HPA axis-related genes corticotropin-releasing hormone (CRH) and neuropeptide Orexin were down-regulated in rats treated by ethanol extract of roots/rhizomes, suggesting that Valeriana jatamansi Jones extract could be involved in exerting effects on dysfunction on HPA axis in anxiety model rats (71).
Iridoid fraction of Valeriana jatamansi Jones (IEFV) (6, 9, 12 mg/kg, for 7 d, p.o.) revealed that IEFV might inhibit the excitability of the central nervous system (CNS) through increasing the GABA level (108). ACPC, a formula (with 39% Valeriana jatamansi Jones) for treating anxiety-related diseases clinically in China, was explored to identify the exact molecular mechanism on the roles in the mediation of anxiety. And it was analyzed that ACPC consisted of hesperidin 1.05 mg/g and Jujuboside A 7.50 mg/g (125,126). Treatment of ICR mice with saline or ACPC (4.8 g/kg, for 10 d, i.p.) could slightly prolong the incubation period of convulsion in mice (92.67±4.03 s) but was not statistically different, as compared with the control group (88.67±3.47 s). However, the anti-anxiety effect of diazepam and ACPC was antagonized by flumazenil. And it is known that γ-aminobutyric acid (GABA), a main inhibitory neurotransmitter in the brain, is mainly mediated by GABA-A receptors. GABA-A is simultaneously a receptor antagonist of flumazenil, and can decrease or reverse its effects of anti-anxiety. Therefore, it was indicated that ACPC might exert anti-anxiety effect (but no sedative effects) via benzodiazepine receptors (GABA receptor) (126,127). Likewise, ACPC (3 g/kg) was also capable of increasing OT% as well as OE% of rats in acute stress, reducing the expression of p-ERK1/2 in the hippocampus (1.5 g/kg) and p-ERK1/2 and p-CREB in the cortex and hippocampus (3 g/kg). Moreover, high-dose (3 g/kg) increased the protein level of brain-derived neurotrophic factor (BDNF) in the cortex (0.54±0.06) and hippocampus (0.55±0.05) of rats, as compared to model group (0.37±0.03 and 0.34±0.04 were in the cortex and hippocampus, respectively), suggesting that the mechanism of ACPC anti-anxiety might involve down-regulation of BDNF and ERK/CREB signal pathway (125).
Anti-tumor activities
Desacylbaldrinal, valtral C and desoxidodidrovaltrate isolated from the roots were shown to exert significant selective cytotoxicity against colorectal cancer cell (HCT-116), with moderate IC50 values ranged 1.7–9.3 µM. Particularly, valtral C could significantly inactivate p-PDK1 in a dose-dependent manner and further strongly suppress the downstream signaling proteins p-Akt/mTOR, thus resulted in autophagosome formation in HCT-116 cells (18). Similarly, Valjatrate E was also found to possess anti-metastatic and anti-invasive abilities on human hepatocellular carcinoma cell (HepG2) in vitro, with dose-dependently down-regulated secretion of matrix metalloprotease-2 (MMP-2) and MMP-9, and thus inhibited migration of tumor cells and suppressed MAPK/ERK signaling pathway (66). It has been shown that IVHD-valtrate, an active derivative of Valeriana jatamansi Jones, could restrain the growth and proliferation of ovarian cancer cell A2780 and OVCAR-3 via blocking entrance of cells into the cycle in the G2/M phase and could further trigger cell apoptosis. Furthermore, the gene expression of p21, p27, p53 and Rb were up-regulated whereas protein expression of Cdc25C, Cdc2, Cyclin B1, E2F1 and Mdm2 were down-regulated, and the cleavage of PARP and caspases were augmented, implying that IVHD-valtrate might exhibit the effect against ovarian cancer via p53-MDM2 signaling pathway (55). The anti-cancer effects of Valeriana jatamansi Jones could be associated with its inhibitory effects on invasion and metastasis. A remarkable anti-proliferation effect was observed in vitro at 400 µg/mL of ethanol extract with evidence of cell cycle arrest at S and G2/M phases. Moreover, the mobility of colon cancer cell (SW480) was reduced noticeably in the scratch test, with the relative moving distance data 1,166.667±175.5942 compared to blank control 1,816.667±132.2876, suggesting that the ethanol extract could exert inhibition of tumor cells through proliferation and the cell arrest cycle (75). F3, a novel active iridoid ester fraction, was assessed to play a significant role in treatment of breast cancer by inhibiting cell growth through mediation of DNA damage including DNA strand breaks and γ-H2AX activation, and what mentioned above might be related closely with ROS accumulation. Meanwhile, p38/JNK/ERK phosphorylation was activated, indicating that mitogen-activated protein kinases (MAPKs) might have relevance to F3-induced cell death (128). At the meantime, it was revealed that the apoptosis-related genes Ets-1, Elk-1, Bax, Apaf-1 and Bcl-2 expression are inconsistently expressed between estazolam-induced anxiety model rats and rats treated by the ethanol extract. Due to the up-regulation of five genes expression, it was inferred that the ethanol extract could be closely associated with the adjustment to a multitude of different cancers under stress (73).
Didrovaltrate, baldrinal and valtrate are derivatives of valepotriates derived from Valeriana jatamansi Jones. After adding the same concentration (33 µg/mL) to the hepatoma cells (HTC line), the viability curve revealed that valtrate became approximately 2 times and 8 times more active than didrovaltrate and baldrinal, respectively. Whereas, the dose-response curves indicated that didrovaltrate (66 µg/mL) could lead to cellular mortality of 60% within the first 4 hours of incubation and reach 100% after 7 hours. Furthermore, didrovaltrate (1.25 mg per mouse, i.p.) could induce observably high percent remissions of the KREBS II ascitic tumors, with the alleviation of the ascites within 48 hours among half of mice and enhancement of a survival time higher than 6 months. In contrast, the mice of control group were all dead within 15±2 d after inoculation of ascetic fluid (52).
Nevertheless, researchers found no significant difference in the body weight in mice administrated with IEFV at a daily dose of 3200 mg/kg during 14 days of the acute toxicity study. The results indicated that 3200 mg/kg was the maximum tolerated dose, which is equal to 2666 times of that of adult clinically. And LD50 value was over 2000 mg/kg body weight. Furthermore, the sub-chronic toxicity study revealed no direct correlation between the hematology and blood biochemical indexes, as well as the toxicity of IRFV and the organ damage (110).
Gastrointestinal disorder
It has been demonstrated for years that Valeriana jatamansi Jones possesses remarkable properties of treatment for gastrointestinal disorder caused by human rotavirus (HRV) like autumnal infantile diarrhea, and it has been made into various pharmaceutical drugs including ACPC, Xianrenzhang-Weikang Capsule and Qiuxieling Mixture. A study has showed that the median cytotoxic concentration (CC50) of Qiuxieling Mixture (mainly composed of Valeriana jatamansi Jones) on SA11 rotavirus-infected African green kidney cell (MA104) was determined as 3.62 mg/mL. Nevertheless, effects on suppression of SA11 rotavirus to attack MA104 cells were shown when the concentration range was 1×10-4 to 10 mg/mL, indicating the role of Valeriana jatamansi Jones in mitigating rotavirus induced diarrhea among children (129). Oligosaccharides are right under the spotlight due to the properties including anti-virus, anti-inflammation, antitumor and improving immunity, and some research on Valeriana jatamansi Jones reported that oligosaccharides appeared to possess the bioactivity of regulating gastrointestinal dysfunction. Verbascoses and galactose were especially rich in this species among 10 oligosaccharides tested, suggesting a sign of its functions of improving intestinal microflora (117). Iridoid (6.23, 12.46, 24.92 mg/kg, for 28 d, i.g.) was helped to decrease the content of 5-HT in serum and colon of chronic stress model rats but was enhanced in hypothalamus. However, a reduction of 5-HT/5-HIAA was observed in serum and colon compared to the model group, suggesting the role of iridoid in treating IBS might be relevant to the mediation of 5-HT from gastrointestinal to the central nervous system (CNS) (130). Research has showed that Valeriana jatamansi Jones-treated tree shrews with diarrhea were all recovered with intestinal villi repaired by degrees and the percentage of recovery is over 95% clinically. The mechanism underlying the treatment might be related to the dysfunction of the immune system, which might be caused by an imbalance between immunocytes and antibodies when infected by virus (13-15,131).
Iridoid 11-ethoxyviburtinal was investigated both in vivo and in vitro. It was used to suppress an intense relaxation on primary cultured rat colon longitudinal muscle cells with administration of 4.04 mM at 30 s. Moreover, it could also suppress the shrinkage effect induced by 5-HT but NE or acetylcholine (Ach), pointing out its significance and role in direct relaxation on the colon longitudinal muscle cells of rats was mediated by 5-HT but M receptor or β receptor (44). Likewise, 11-ethoxyviburtinal (1.2 mg/kg, i.g.) decreased the number of 5-HT positive immunoreactive colonic enterochromaffin cells (EC) (1.89±0.78) and reduced apparently the number of colonic mast cells (MC) (1.56±0.73) in IBS rats, as compared to model group (3.67±2.01 and 2.78±1.30 were the number of EC and MC, respectively). Therefore, it is reasonable to assume that 11-ethoxyviburtinal may improve IBS symptoms via reducing the number of positive cells in colonic EC, inhibiting the expression of MC activity, decreasing the release of 5-HT and restoring the abnormal peripheral 5- HT level to normal (7).
Anti-virus activities
A study has showed that CC50 of Qiuxieling Mixture (mainly composed of Valeriana jatamansi Jones) was determined as 3.62 mg/mL on SA11 rotavirus-infected African green kidney cell (MA104). Nevertheless, effects on suppression of SA11 rotavirus to attack MA104 cells were shown when the concentration range is 1×10-4-10 mg/mL (129).
Furthermore, a novel method bioassay-guided separation was utilized with a fission yeast Schizosaccharomyces pombe (S. pombe), was helped to separate valtrate (1). Valtrate (1) was found to possess anti-HIV activity in the way of suppressing Rev-transport from the nucleus to cytoplasm. And it displayed 44% inhibition on HIV-1-p24 production at the concentration of 0.5 µM with no cytotoxicity against the host MT-4 cells, still, it could entirely inhibit the Rev-transport of the fusion protein in S. pombe from the nucleus to cytoplasm at the concentration of 3 mg/mL (132). Another similar study reported that the epoxy section would be the decisive function to display the bioactivity of valtrate (1). Hence another method bioisostere was utilized to search for the bioisosteres of valtrate (1) and thus to search for new anti-HIV agents. In this way, Tamura, S. et, al demonstrated that analog 2 (5,6-dihydrovaltrate), designed by the MO calculation, could entirely inhibit Rev-transport at the concentration of 10 µM, indicating that this unprecedented mechanism of action would be helpful to explore more potent analogs to inhibit exportation of Rev protein acting as anti-HIV agents (67). Among water, methanol and chloroform extracts, a notable reduction of hepatitis C virus (HCV) replication was found only in Huh-7.5 cells treated with methanol extract of the plant in a dose-dependent manner. Methanolic sub-fraction F4 was indicated to be the most poisonous with CC50 value 251.28±0.47 µg/mL and was observed to suppress HCV by 30%-70% and decrease the expression of NS5B about 50% (84). After applying ACPC (with 39% Valeriana jatamansi Jones) for treating infants infected with HRV, it was obviously found that serum levels of immunoglobulins and complements were changed. It was observed that CD3%, CD4% and NK cell were increased with potent effect on diarrhea (13). Similarly, another report showed that applying Qiuxieling Mixture (mainly composed of Valeriana jatamansi Jones) for treating infants infected with HRV was helpful to notice apparent change before and after treatment. It was observed that lower levels of serum IgG, IgA, IgM, C3, CD3+, CD4+, CD4+/CD8+ ratio, and higher levels of CD19+ and CD8+ after treatment with apparently improved immune functions. And it should be noticed that great efficacy (95.3%) were observed after treatment comparing with the normal treatment group with Montmorillonite and Bifidobacterium (79.5%), respectively (131).
Anti-oxidant and anti-microbial activities
There are strong signs of anti-oxidant activities with more unstable constituents isolated from roots and rhizomes of the species. A recent study showed that a comparison between the roots of Valeriana jatamansi Jones from the natural environment and planted in Indian west Himalaya indicated higher total flavonoids, phenols and apparent antioxidant activity was noted from the planted source, but a less tannin content and antioxidant effect in DPPH (20). In another respect, however, three iridoid valepotriates (acevaltrate, valtrate and 1-β acevaltrate) were performed in a forced degradation study. A reduction of the capacity of scavenging free radical, cytotoxicity and cell apoptosis were observed after the degradation of the iridoid valepotriates. Thus, it was assumed that conceivable mechanism of antioxidant activities might be related to GABAergic signaling pathways (45). The 50% methanolic extracts obtained from the whole plant showed moderate radical scavenging activity but no prevention of oxidative deoxyribonucleic acid (DNA) damage (85). What’s more, another study on orally administration of Valeriana jatamansi Jones rhizome extract (VRE) into PD mice was performed. With the evidence of amelioration of LPO, ROS and improvement of antioxidants levels in PD mice, it was effectively demonstrated that neuroprotective effect of VRE might be possibly achieved through its ability to improve the antioxidant activities and attenuate neuroinflammation (88). Furthermore, ISSR markers were utilized to compare the antioxidant activity with ABTS, DPPH and TRAP among five distantly located populations of Valeriana jatamansi Jones. The results implied that an apparent variation across the population is found and only 3/97 ISSR markers with high mean genetic diversity (HB8-5, HB12-1 and 17898B-8) were positively related to antioxidant activity in DPPH assay (27).
Valeriana jatamansi Jones and its major compounds possess noteworthy antimicrobial activities. 8-acetoxyl-pathchouli alcohol, a kind of sesquiterpenoid, showed slight inhibitory activity against Pseudomonas aeruginosa (64 µg/mL minimum inhibitory concentration, MIC) and Staphylococcus aureus (128 µg/mL MIC) compared to gentamicin (5 µg/mL MIC), and exhibited the moderate activities against B16 with IC50 value of 31.43 µg/mL compared to cisplatin (15.25 µg/mL) (63). Similarly, the essential oil at the concentration of 400 µg/mL appeared to exhibit antifungal effect against Microsporum canis and Aspergillus flavus with 50% and 60% inhibition respectively, and to inhibit the growth of Fusarum solani to 70%. Notably, essential oils of Valeriana jatamansi Jones at the concentration of 400 µg/mL showed 70% antifungal activity against F. solani (96). The aerial parts with different extracts were shown to exert adverse effects on S. aureus (chloroform fraction, 0.27 mg/mL MIC), Bacillus subtilus (hexane fraction, 0.31 mg/mL MIC) compared to control imipenem (less than 0.0005 mg/mL MIC), and M. canis (crude extract, 0.54 mg/mL, chloroform fraction, 0.36 mg/mL, n-Hexane fraction, 0.19 mg/mL) compared to miconazole (0.0001 mg/mL) as well as A. flavus (chloroform fraction, 0.69 mg/mL) compared to Amphotericin B (0.0002 mg/mL). And the hexane fraction was found to be the most potential inhibitor against M. canis, with an MIC value of 0.19 mg/mL as compared to miconazole (0.0001 mg/mL) (133). Both Miao nationality medicine compound wolailiu solution (composed of Valeriana jatamansi Jones, Cinnamomum cassia Presl, Ramulus Mori, Pinus armandi Franch, etc.) exhibited completely inhibitory effect on S. aureus, B. subtilis and Candida albicans in vitro (134). Powder and essential oil of Valeriana jatamansi Jones exhibited inhibitory effect on S. aureus (0.625 mg/mL and 6.25 mg/mL MIC), B. subtilis (2.5 mg/mL MIC), E. coli (2.5 mg/mL MIC) and C. albicans (5 mg/mL MIC) in vitro (98,135).
Hepatoprotective activity
One study was reflected that a reduction of serum triglyceride (TG), an increase of serum high-density lipoprotein cholesterol and the slowness of weight gain were observed in hyperlipidemia rats fed with the iridoids rich fraction of Valeriana jatamansi Jones (IRFV) in different dosages (7.5, 15, 30 mg/kg/d, for 20 d). Of note, IRFV activated the expression of peroxisome proliferator-activated receptor α protein but decreased sterol regulatory element-binding proteins and liver X receptor α protein in the liver to increase the expression of apoprotein A5, and augmented the activities of lipoprotein lipase, hepatic lipase to metabolize more TG to regulate the lipid metabolism to exert its role in hepatoprotective effect (109). Furthermore, the ethanol extract of Valeriana jatamansi Jones decreased TG level in plasma and liver, total cholesterol level in the liver as well as alanine aminotransferase level in plasma of hyperlipidemia rats, thus confirming the ethanol extract’s involvement in hepatoprotective effect against hyperlipidemia (9). Orally administrated with hydroalcoholic extract of the plant for 9 weeks effectively reversed the augmented levels of γ-glutamyl transferase and alkaline phosphatase. And various biochemical markers of hepatic injury were also affected. For instance, serum alkaline phosphatase levels and lipid peroxidation were downregulated, but the effects on glutathione and serum γ-glutamyl transferase were not statistically significant, and drug-metabolizing enzymes xanthine oxidase and Glutathione S-transferase were decreased in treated model rats, thus hydroalcoholic extract were presumed to exert the effect of ameliorating the liver cirrhosis in model rats (82). In addition, administration of the aqueous extract of roots of the species (VJAE) (300, 500 mg/kg, for 7 d, p.o.) in CCl4-induced hepatotoxicity albino rats was investigated. The histopathological examination results implied that inflammatory infiltrates and hepatic architecture were disrupted in most of the field with VJAE 300 mg, whereas 500 mg was superior to 300 mg due to the elevated levels of catalase, indicating the antioxidant activity of VJAE was involved in the hepatoprotective activity (101).
Cardiovascular disorder
An experiment in vitro presented the results that the mean arterial blood pressure of normotensive anaesthetized rats administrated with the crude ethanol extract of Valeriana jatamansi Jones rhizome (Vjj.Cr) intravenously was fallen dose-dependently but partially reversed by glibenclamide. Furthermore, a selective and glibenclamide-sensitive relaxation of high K+ (80 mM)-induced contractions were partially suppressed by the chloroform and aqueous fractions but it was totally suppressed in low K+ (20 mM)-induced contractions of rabbit aorta preparations, illustrating that blood pressure lowering activities was mediated probably via ATP-dependent K+ channel activation but not calcium channel blockade, the characteristic of the extract might help treat cardiovascular disorder (92). Another report validated the fact that the ethyl acetate fraction of this plant could reduce the cardiac contractility and frequency, but to a lesser extent in the coronary outflow of the rabbit heart preparation (78).
Analgesic, sedative and antispasmodic activities
Different extracts of the species testing analgesic activity conducted by hot-plate, radiation heat radiation heat stimulation, moving and lifting dual anterior limbs were observed and were compared with the water decoction. The results were inferred that the water decoction could significantly affect analgesia and could positively stimulate the intestinal muscle of rabbits in vitro. Nevertheless, a discernible effect of that mentioned above was observed in mother liquor, with a weak activation in 1-butanol section, but an indistinction in acetic ether and petroleum ether section, implying the potential effect of analgesia and gastrointestinal regulation (107). Moreover, flavonoids extract was also shown to exert analgesic activity revealed by the hot-plate and acetic acid writhing test in the intestine of rabbit in vitro (111). What’s more, 6-methylapigenin (MA), a bioactive flavonoid isolated from the roots and rhizomes, might be acting as tranquilizer with the evidence of its ability to act as a ligand for the benzodiazepine binding sites (118). Moreover, MA and 2S(-)-hesperidin (HN) were further investigated on their potential sedative and sleep-enhancing properties. However, administration of HN (2 mg/kg, i.p.) in rats exhibited significant improvement on thiopental-induced sleeping time, whereas MA (1 mg/kg) alone was failed to discover its sedative and sleeping-inducing properties but enhanced notably in the combination of HN (2 mg/kg), suggesting MA was potential to enhance the sedative properties of HN (30). In addition, the crude extract of Valeriana jatamansi Jones rhizome (Vjj.Cr) and its fractions exerted negligible inhibitory effect against high K+ (80 mM)-induced contractions in rabbit jejunum and guinea pig ileum in vitro but caused complete relaxation against low K+ (20 mM)-induced contractions, implying the spasmolytic effect was probably mediated through a KATP channel opener but not calcium channel blockade (92). Besides, the chemotype (patchouli alcohol) extract (DCME) and essential oil (VPAEO) (40 and 80 mg/kg, p.o.) inhibited the number of writhings but exerted no difference in tail-flick model, reflecting that merely peripheral analgesic activity but not higher center was involved. And the effect of aspirin in acetic writhing was no potentiation seen in DCME, but was activated in VPAEO, suggesting the underlying mechanism of analgesic activity was possibly mediated by KATP channel activation other than cyclooxygenase (COX) inhibition (92,99). The ethanol extract of the species could effectively prolong the convulsion latent period to a certain extent, but without influence on the rate of convulsion and could significantly decrease the death rate of pentylenetetrazol (PTZ)-induced convulsion mice at the dose of 2.0 g/kg (i.g. for 6 d). And it should be noticed that GABA level of the brain was increased with the administration of the extract, thus, the relationship between the expression of GABA and anti-convulsion activity became manifest (76). Another mechanism of treating epilepsy is also relevant to this species. Valepotriate isolated from the species increased the expression of Bcl-2, GABAA and GAD65 proteins, and decreased the expression of caspase-3 protein, whereas exerted negligible activity on the expression of GABAB in maximal electroshock-induced seizure and PTZ-induced epilepsy rats, illustrating the positive effect of valepotriate on epilepsy might be related to the regulation of GABA and suppression of neuronal apoptosis (6).
Anti-inflammatory and Immunoregulatory Activities
The hydroalcoholic extract of leaves with the presence of saponin, glycosides, flavonoids, tannins and phenolic compounds, etc. appeared to exert the inhibition of inflammatory activity (79). The aqueous (Aq-Ext) and methanolic (Me-Ext) rhizomes extracts were revealed to suppress inflammatory activity in carrageenan-induced paw oedema rats, but only Aq-Ext was shown dose dependently suppressed. Moreover, the curative effect resembled those observed in non-steroidal anti-inflammatory drugs (90). Drug serum of the herb (powder of the plant) could not only effectively enhance the proliferation and transformation rate of splenic lymphocytes with ConA and LPS combination, but could also promote the energy metabolism activity of peritoneal macrophage in mice, reflecting the drug serum of the herb could activate the specific humoral immune function and cellular immune response of conductors (136). What’s more, diminished levels of pro-inflammatory cytokines (TNF-a, IL-1β, IL-2 and IL-6) were significantly ameliorated following Valeriana jatamansi Jones rhizome extract (VRE) treatment in MPTP-induced PD mice, providing conclusive evidence of the potential of VRE to mitigate inflammatory damage in PD (88). A notable anti-inflammatory-like activity (0.14% edema inhibition rate) of the methanolic extract was observed at a dose of 200 mg/kg in carrageenan-induced paw edema model compared to diclofenac (0.23%). The crude methanolic leaf extract of the species was applied in carrageenan-induced hind paw edema rats (CHPER) and validated its ethnopharmacological uses in anti-inflammation via significant inhibition of inflammation at early and late phases as compared with NSAID product diclofenac (133). Likewise, the leaves and ethyl acetate fraction of the species in treatment for CHPER were also investigated in vitro and in vivo, indicating its weak inflammatory activity compared to standard (piroxicam gel 0.5%) at initial stages, but it revealed a comparable effect with that of standard at later phases of inflammation (86). And it was similarly investigated that orally administrated Tagara (made into capsules) into the same model at dose of 40 mg/kg bd exhibited anti-inflammatory property with dose-dependently but not the larger dosages of 80, 120 mg/kg (137). When applying ACPC (with 39% Valeriana jatamansi Jones) and Qiuxieling Mixture (mainly composed of Valeriana jatamansi Jones) for treating infants infected with HRV, it was obviously found that serum levels of immunoglobulins and complements were changed, with lower levels of IgG, IgA, IgM, C3, CD3+, CD4+, CD4+/CD8+ ratio, and higher levels of CD19+ and CD8+. And it should be noticed that the immune functions were apparently improved after treatment (13,131).
Insecticidal, acaricidal, nematicidal and antileishmanial activities
The essential oil isolated from the whole plant of the species was active against pests Aphis cracccivora Koch with LC50 values of 90.75 ppm at 24 h contact and 57.03 ppm at 48 h contact (37). The root extract of the herb (BAL-O) was analysed that the major constituents are aristolene (5.19%), cadinol (5.23%), caryophyllene oxide (5.46%), cubenol (5.97%), patchouli alcohol (8.55%) and 2-butanone,4-(2,6,6-trimethyl-2-cyclohexen-1-yl) (10.11%). And it was revealing that LC50 values of BAL-O against larvae of 5 mosquito species including Anopheles culicifacies, Aedes aegypti, Aedes albopictus, Anopheles stephensi and Culex quinquefasciatus are 42.8, 51.2, 53.8, 68.1 and 80.6 mg/liter, respectively (95). Moreover, fumigant of the essential oils was investigated to study their activity against Japanese termites (Reticulitermes speratus Kolbe). The essential oil was revealed to exert moderate or weak fumigant toxicity (mortality ranged 2% -88%) after treatment for 2 days. Whereas, among the chemical compositions, only cis-asarone (88.82%) and cis-ocimene (0.12%) showed 100% toxicity against Japanese termites after treatment for 7 days, which might be due to the lower vapor pressure, suggesting an importance of exposure time and vapor pressure in evaluating the essential oils. Besides, comparing with the other components of Liquidambar orientalis, it was concluded that the insecticidal activity against Japanese termites was possibly due to a benzene ring existed in the active compounds (124).
Another pest pine wood nematode (Bursaphelenchus xylophilus) applied with the valerian oils was also reported. It was indicated that the oils containing 4 major compounds cis-ocimene, linalool, trans-asarone, cis-asarone exhibited apparent nematicidal activity against B. xylophilus mainly due to the contents of cis-asarone (34).
Another area of the activity against Leishmania spp was investigated explicitly. The methanol (VjM) and chloroform (VjC) extracts of the valerian roots were shown to exert anti-viability activity of promastigotes and amastigotes of L. major as well as L. donovani promastigotes. F3, the most active fraction obtained from VjC, showed IC50 at 3-7 µg/mL against the promastigotes and 0.3 µg/mL against intracellular amastigotes of L. major, and induced apoptotic death in leishmanial cells through analysis of cytotoxicity, cellular morphology, phosphatidyl serine, oligonucleosomal DNA fragmentation, mitochondrial membrane and ROS generation (89). And Glaser et al. further identified 3 valtrates and 3 cinnamic acid derivatives as structures responsible for antileishmanial principles (IC50 0.8-5.8 µg/mL) with bioassay-guided fractionation (68).
Cytotoxicity
Cytotoxicity against various cancer lines of compounds and extracts of the herb are summarized in Table 3. Preliminary pharmaceutical analysis revealed that quite a number of compounds isolated from Valeriana jatamansi Jones could effectively exhibit cytotoxicity against various cancer cell lines, i.e., human small-cell lung cancer (GLC4), human colorectal cancer (COLO 320), lung adenocarcinoma (A549), gastric carcinoma cells (SGC 7901), metastatic prostate cancer (PC-3M), hepatoma (Bel7402) and colon cancer (HCT-8) cell lines with IC50 values ranging from 0.89 to 9.76 µM, and some of the compounds like valepotriates (valtrate and didrovaltrate) and iridoids (3,8-epoxy iridoids) displayed toxicity against neoplastic cells, with IC50 values of 0.4-15.2 µM (31,35,42,46,48,69). Valejatanin A, a diene iridoid, showed inhibition of human colon carcinoma cell (HT29), human leukemia cell (K562) and mouse melanoma cell (B16) with IC50 values of 14.37, 2.85 and 15.25 µg/mL, respectively (63). Treatment of compound 5-7, derived from the roots, could exert significant selective cytotoxicity against colorectal cancer cell (HCT-116), with IC50 values ranging from 1.7 to 9.3 µM (18). Besides, five iridoids and six analogues were shown to display no dramatic cytotoxicity effect on A549 and SGC 7901 except 3,8-epoxy and 4-acetoxy iridoids (42,139). Nevertheless, researchers found no significant difference in the body weight in mice administrated with IEFV at a daily dose of 3200 mg/kg for 14 days of the acute toxicity study. They indicated 3200 mg/kg was the maximum tolerated dose, which was equal to 2666 times of that of adult clinically. And LD50 value was over 2000 mg/kg body weight. Furthermore, the sub-chronic toxicity study revealed no direct correlation between the hematology and blood biochemical indexes, as well as the toxicity of IRFV and the organ damage (110).
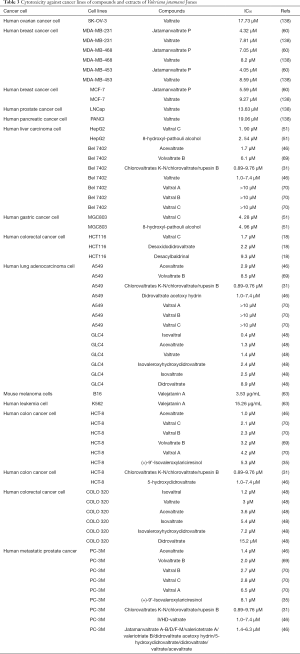
Full table
Other pharmacological activities
Extracts and essential oils of the herb were shown to exhibit strong peripheral and weak central antinociceptive effect, which might be probably acting through inhibition of prostaglandin synthesis and opioidergic pathway (91).
The aqueous extract of the root mainly contain hesperdin (HN) countered radiation-induced free radicals after exposed to 5 Gy γ-irradiation for 4 h, decreased mitochondrial mass, and enhanced reproductive viability of SV-40 transformed human embryonic lung fibroblast (MRC5 V1) cells against radiation-induced naked DNA damage in solution (103).
What’s more, the crude extract of Valeriana jatamansi Jones (Vj.Cr) caused entire relaxation by low K+ (25 mM)-induced shrink, whereas mildly inhibited the contractions by high K+ (80 mM)-induced shrink in guinea-pig trachea. Of note, it was presented that the suppression of low K+ (25 mM)-induced shrink was reversed with the presence of glibenclamide (3 µM), and the contractions induced by low K+ was relaxed glibenclamide-sensitively caused by cromakalim, while no effect on high K+-induced shrink. Therefore, with the combination of earlier study they reported (92), it was suggested that this species possessed bronchodilatory action through the cooperation of prevailing ATP-denpendant K+ channel activation and negligible Ca2+ entry blocking mechanism.
Related mechanisms
More recent research on Valeriana jatamansi Jones is facilitating to explore predominant functions of 6-methylapigenin, valeric acid, valepotriate and different extracts, especially their roles playing in regulating GABAergic activity (6,65,108). Reduction of the capacity of scavenging free radical, cytotoxicity and cell apoptosis were observed after the degradation of the iridoid valepotriates. Thus, it was assumed that conceivable mechanism of antioxidant activities of iridoid valepotriates might be related to GABAergic signaling pathways (45). Valepotriate isolated from the medicinal herb increased the expression of GABAA, whereas exerted negligible activity on the expression of GABAB in seizure and epileptic rats, illustrating the positive effect of valepotriate on epilepsy might be related to the regulation of GABA (6). What’s more, 6-methylapigenin (MA), a bioactive flavonoid isolated from the rhizomes and roots, might be acting as tranquilizer with the evidence of its ability to act as a ligand for the benzodiazepine binding sites (118). The results of administration of the rhizomes extract and valeric acid isolated from the herb were revealed that both of the administration could decrease the level of lipid peroxidation and reduce the restored GSH in brains. It is noteworthy that the effect of improving memory with the administration of valeric acid (40 mg/kg) was significant compared with the plant extract. The study might help validate the fact of neuroprotective potentiality of the herb and its isolate valeric acid in ameliorating memory cognition and retention via GABAergic activity in model dementia rats (65). Another in vivo study revealed that iridoid fraction of Valeriana jatamansi Jones (IEFV) (6, 9, 12 mg/kg, for 7 d, p.o.) might inhibit the excitability of the central nervous system (CNS) through increasing the GABA level (108). ACPC was explored to identify the exact molecular mechanism on the roles in the mediation of anxiety (125,126). What’s more, treatment of ICR mice with saline or ACPC could slightly prolong the incubation period of convulsion in mice but was not statistically different, as compared with the control group. However, the anti-anxiety effect of diazepam and ACPC was antagonized by flumazenil. Therefore, it was indicated that ACPC might exert anti-anxiety effect (but no sedative effects) via GABA receptor (126,127).
It has been identified that the suppression of GABA system could help activate the hypothalamus-pituitary-adrenal (HPA) axis at the level of paraventricular hypothalamic nucleus (140). The ethanol extract and total valeportriate from roots and rhizomes were revealed to play a significant role in antianxiety activity and sedation and hypnogenesis. And after the animals were orally administrated with valtrate (for 10 d), serum CS and the brain hippocampal tissue in the neurotransmitter 5-HT, DA and NE levels were down-regulated, suggesting that the ethanol extract and total valeportriate from the herb could play a role in regulation of anxiety through HPA axis system and could be a potential anxiolytic drug (8,113). To further study the dysfunction of HPA in anxiety model rats, Yan et, al. found that blood β-EP and CS levels were higher in the anxiety model group rats than in the normal group, and the expression of HPA axis-related genes CRH and neuropeptide Orexin were down-regulated in rats treated by ethanol extract of roots/rhizomes, suggesting that ethanol extract could be involved in exerting effects on dysfunction on HPA axis in anxiety model rats (71).
Therefore, it could be noted that the mechanisms between GABAergic activity and HPA axis have not been identified yet.
Future prospects and conclusions
Crude plant of Valeriana jatamansi Jones contains various compounds and a wide range of pharmacological activities. In this regard, valepotriates could play a momentous role for the reasons that they are not only a predominant proportion of constituents in the herb, but also these constituents are relatively bioactive and well-studied compared to other derivatives. However, chemical compounds responsible for these effects are still unknown, though the preliminary bioactivities of a few major compounds (valerian, IVHD-valtrate, 11-ethoxyviburtinal, and hesperidin) have been extensively studied. In fact, it is a primary raw ingredient of the health product Tagara for treating depressive insomnia. Clinically, polyherbal combination with the herb ACPC and Compound Mati Xiang Keli have been applied for the treatment of anxiety and PRE, respectively. The mechanism of the formula for treating the diseases might attribute to the complicated structural constituents, and the exact mechanism of the formula need to be further investigated. For example, it was indicated that the aqueous extract (containing hesperidin) was able to protect against radiation-injury in vitro. While the radioprotective efficacy of the aqueous extract cannot be conducive solely to the hesperidin content alone, suggesting other compounds present in the extract must conduce to its radioprotective ability (103). Therefore, further studies are warranted to illuminate the mechanisms responsible for these activities at the molecular levels but not just in preliminary studies, which is a major challenge for us to promote its usages clinically. However, it could be noted that the mechanisms between GABAergic activity and HPA axis have not been identified yet. As intensive research has indicated the dual-function of antitumor and anti-inflammatory effects of the natural compounds, great attention of cancer treatment should be paid. Nevertheless, the anticancer effects were not strong enough to satisfy the therapy, therefore, molecular modification of the compounds will be helpful. As an important herbal medicine with great potential to be explored, another work such as the exact mechanism of how the bioactive compounds exert therapeutic effect on specific diseases and proper application of polyherbal combination should be studied further. Moreover, sometimes the names of chemical compounds are imprecise like Jatamanvaltrate P with different molecular formula (C27H40O10 and C22H32O9, compound NO.46 and NO.47 in Table 1) (60,61) that might be obscure when applied into use. The acute and sub-chronic toxicity tests have provided the present risks of the potential drug dose-response relationship and toxic characteristics, as well as the biological transport and biotransformation of compounds in the organism.
Meanwhile, it should be noted that pharmacokinetics and pharmacodynamics of the herb have been hardly studied, thus the process of the absorption, metabolism, decomposition, excretion and side effects after the chemicals delivered into the body should be the next research direction, and this should be noticed by other advanced studies or clinical trials to avoid adverse effects. Moreover, novel methods such as bioassay-guided separation, bioisostere and ISSR markers should be utilized to explore more latent components with bioactivity. These unprecedented mechanisms of action mentioned in section “Related Mechanisms” would be helpful to explore more potent analogs to be specific diseases-treating agents, which will be contributed to improving efficiency of exploration at the same time. On the other hand, the improvement of the quality control of Valeriana jatamansi Jones crude drugs and related latent chemicals with bioactivities could be utilized by the chemical markers tabbed by identified major bioactive structure. Furthermore, bioactive compounds in large-scale production could be achieved with the metabolic engineering, which could be simultaneously helpful to protect the species naturally occurring in the wild.
This review comprehensively summarizes research progress in botanical characteristics, pyhtochemistry and pharmacology of Valeriana jatamansi Jones to provide a strong bolster for a promising natural resource of drugs for the treatment of various diseases and a long term development in exploring highlighted area.
Acknowledgments
The authors thank the members of Yunnan Minzu University-Hong Kong Baptist University Joint Laboratory of Traditional Natural Medicine, including Baomin Fan, Guangzhi Zeng, Junlin Yin, Yongyun Zhou, Jingchao Chen, Weiqing Sun, Zhenxiu He and Chunju Yang for their great help and support.
Funding: This research was funded by National Natural Science Foundation of China, grant number 31660335 and Key-Area Research and Development Program of Guangdong Province, grant number 2020B1111110003.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/lcm-20-54
Peer Review File: Available at http://dx.doi.org/10.21037/lcm-20-54
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-20-54). CL serves as an unpaid editorial board member of Longhua Chinese Medicine from Nov 2020 to Oct 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dhiman B, Sharma P, Pal PK. Biology, chemical diversity, agronomy, conservation and industrial importance of Valeriana jatamansi: A natural sedative. J Appl Res Med Aromat Plants 2020;16:100243 [Crossref]
- Wang C, Zheng Z, Deng X, et al. Flexible and powerful strategy for qualitative and quantitative analysis of valepotriates in Valeriana jatamansi Jones using high-performance liquid chromatography with linear ion trap Orbitrap mass spectrometry. J Sep Sci 2017;40:1906-19. [Crossref] [PubMed]
- Ankush K, Susheel V, Puneet S. Stylar movement in Valeriana wallichii DC. - A contrivance for reproductive assurance and species survival. Curr Sci 2011;100:1143-4.
- Prakash V. Indian Valerianaceae-A monograph on a medicinally important family. Scientific Publishers,1999.
- Jugran AK, Rawat S, Bhatt ID, et al. Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phytother Res 2019;33:482-503. [Crossref] [PubMed]
- Wu A, Ye X, Huang Q, et al. Anti-epileptic Effects of Valepotriate Isolated from Valeriana jatamansi Jones and Its Possible Mechanisms. Pharmacogn Mag 2017;13:512-6. [Crossref] [PubMed]
- Xiao C, Tao S, Yan X, et al. Influences of 11-ethoxyviburtinal of Valeriana jatamansi Jones on the expression of enterochromaffin cells and mast cells in rats with irritable bowel syndrome. Journal of Beijing University of Traditional Chinese Medicine 2016;39:21-5.
- Shi SN, Shi JL, Liu Y, et al. The anxiolytic effects of valtrate in rats involves changes of corticosterone levels. Evid Based Complement Alternat Med 2014;2014:325948 [Crossref] [PubMed]
- Chen C, Yan Z, Li S, et al. Effect of the Extract of Valeriana jatamansi on the Blood Lipid and Liver Function in Experimental Hyperlipidemia Rats. Chinese Journal of Experimental Traditional Medical Formulae 2012;18:154-7.
- Fernández S, Wasowski C, Paladini AC, et al. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol Biochem Behav 2004;77:399-404. [Crossref] [PubMed]
- Li Y, Chen C, Mao S, et al. Anti-depression Effect and Mechanism of Valerianae Jatamansi Rhizoma et Radix. Chinese Journal of Experimental Traditional Medical Formulae 2020;26:235-40.
- Bai JS, Zhang QC, Guo DD, et al. Clinical study on compound prescription with Valerianae Jatamansi Rhizoma et Radix in treatment of generalized anxiety disorder. Zhongguo Zhong Yao Za Zhi 2017;42:4888-92. [PubMed]
- Li F, Xie J, Du Z. Clinical Observation of Compound Mati Xiang Keli in the Adjuvant Treatment of Infantile Rotavirus Enteritis. CJITWM 2005;25:762.
- Ma J, Chen S, Xie X. The study of curative effects of treatment rotavirus enteritis with Vaieriana Jatamansi Jone (VJJ). Journal of Kunming Medical University 1987;4:78-81.
- Pang Q, Wan X, Chen S, et al. Treatment of rotavirus infection in tree shrews (Tupaia Belangeri Yunalis) with Herba Valeriana Jatamansi (VJ). Journal of Traditional Chinese Medicine 1984;56-61.
- Purohit S, Rawat V, Jugran AK, et al. Micropropagation and genetic fidelity analysis in Valeriana jatamansi Jones. Journal of Applied Research on Medicinal and Aromatic Plants 2015;2:15-20. [Crossref]
- Chen R. Shoot organogenesis and somatic embryogenesis from leaf explants of Valeriana jatamansi Jones. Scientia Horticulturae 2014;165:392-7. [Crossref]
- Tan YZ, Peng C, Hu CJ, et al. Iridoids from Valeriana jatamansi induce autophagy-associated cell death via the PDK1/Akt/mTOR pathway in HCT116 human colorectal carcinoma cells. Bioorg Chem 2019;87:136-41. [Crossref] [PubMed]
- Liu K, Yang J, Luo X, et al. Determination of Chlorogenic Acid and Total Phenolic Acid Content in Valeriana jatamansi. Hubei Agricultural Sciences 2017;56:288-90.
- Bhatt ID, Dauthal P, Rawat S, et al. Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Valeriana jatamansi Jones. Scientia Horticulturae 2012;136:61-8. [Crossref]
- Tang YP, Liu X, Yu B. Two new flavone glycosides from Valeriana jatamansi. J Asian Nat Prod Res 2003;5:257-61. [Crossref] [PubMed]
- Raina AP, Negi KS. Essential Oil Composition of Valeriana Jatamansi Jones from Himalayan Regions of India. Indian J Pharm Sci 2015;77:218-22. [Crossref] [PubMed]
- S Z. Comparative Study on Analgesic Components Effects of Valeriana Jatamansi Jones from Different Habitat. Guizhou University, 2019.
- Baokang H. Studies on Pharmacognosy of Valeriana L. and Intraspecific Variation of Valerian in China. School of Pharmacy Second Military Medical University, 2005.
- Jinli S. Study on the Medicinal Plant Resources of Valeriana in China. Beijing University of Chinese Medicine, 2004.
- Jugran AK, Bhatt ID, Rawal RS, et al. Patterns of morphological and genetic diversity of Valeriana jatamansi Jones in different habitats and altitudinal range of West Himalaya, India. Flora-Morphology, Distribution Functional Ecology of Plants 2013;208:13-21. [Crossref]
- Jugran A, Rawat S, Dauthal P, et al. Association of ISSR markers with some biochemical traits of Valeriana jatamansi Jones. Ind Crops Prod 2013;44:671-6. [Crossref]
- Sundaresan V, Sahni G, Verma RS, et al. Impact of geographic range on genetic and chemical diversity of Indian valerian (Valeriana jatamansi) from northwestern Himalaya. Biochem Genet 2012;50:797-808. [Crossref] [PubMed]
- Jugran AK, Bahukhandi A, Dhyani P, et al. Impact of Altitudes and Habitats on Valerenic Acid, Total Phenolics, Flavonoids, Tannins, and Antioxidant Activity of Valeriana jatamansi. Appl Biochem Biotechnol 2016;179:911-26. [Crossref] [PubMed]
- Marder M, Viola H, Wasowski C, et al. 6-Methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS. Pharmacol Biochem Behav 2003;75:537-45. [Crossref] [PubMed]
- Lin S, Zhang Z, Chen T, et al. Characterization of chlorinated valepotriates from Valeriana jatamansi. Phytochemistry 2013;85:185-93. [Crossref] [PubMed]
- Li S, Yan ZY. Research of iridoids from Valeriana jatamansi Jones. Chinese Journal of New Drugs 2012;21:633-7.
- Verma RS, Verma RK, Padalia RC, et al. Chemical diversity in the essential oil of indian valerian (Valeriana jatamansi Jones). Chem Biodivers 2011;8:1921-9. [Crossref] [PubMed]
- Kim J, Seo SM, Lee SG, et al. Nematicidal Activity of Plant Essential Oils and Components from Coriander (Coriandrum sativum), Oriental Sweetgum (Liquidambar orientalis), and Valerian (Valeriana wallichii) Essential Oils against Pine Wood Nematode (Bursaphelenchus xylophilus). J Agric Food Chem 2008;56:7316-20. [Crossref] [PubMed]
- Lin S, Chen T, Liu XH, et al. Iridoids and lignans from Valeriana jatamansi. J Nat Prod 2010;73:632-8. [Crossref] [PubMed]
- Das J, Mao AA, Handique PJ. Terpenoid compositions and antioxidant activities of two Indian valerian oils from the Khasi Hills of north-east India. Nat Prod Commun 2011;6:129-32. [Crossref] [PubMed]
- Tewary DK, Bhardwaj A, Shanker A. Pesticidal activities in five medicinal plants collected from mid hills of western Himalayas. Ind Crops Prod 2005;22:241-7. [Crossref]
- Dong FW, Liu Y, Wu Z, et al. Iridoids and sesquiterpenoids from the roots of Valeriana jatamansi Jones. Fitoterapia 2015;102:27-34. [Crossref] [PubMed]
- Ming DS, Yang YY, He CH. The structures of three novel sesquiterpenoids from Valeriana Jatamansi Jones. Tetrahedron Letters 1997;38:5205-8. [Crossref]
- Xu J, Yang B, Guo Y, et al. Neuroprotective bakkenolides from the roots of Valeriana jatamansi. Fitoterapia 2011;82:849-53. [Crossref] [PubMed]
- Tan YZ, Yong Y, Dong YH, et al. A new secoiridoid glycoside and a new sesquiterpenoid glycoside from Valeriana jatamansi with neuroprotective activity. Phytochem Lett 2016;17:177-80. [Crossref]
- Wang RJ, Chen HM, Yang F, et al. Iridoids from the roots of Valeriana jatamansi Jones. Phytochemistry 2017;141:156-61. [Crossref] [PubMed]
- Xu J, Zhao P, Guo Y, et al. Iridoids from the roots of Valeriana jatamansi and their neuroprotective effects. Fitoterapia 2011;82:1133-6. [Crossref] [PubMed]
- Zhang T, Hu J, Wang J, et al. Effects of ethoxyviburtinal-11 on the contractile response of rat’s colon longitudinal muscle cells and its mechanism. China Journal of Traditional Chinese Medicine and Pharmacy 2016;31:1386-9.
- Wang F, Zhang Y, Wu S, et al. Studies of the structure-antioxidant activity relationships and antioxidant activity mechanism of iridoid valepotriates and their degradation products. PLoS One 2017;12:e0189198 [Crossref] [PubMed]
- Lin S, Shen YH, Li HL, et al. Acylated Iridoids with Cytotoxicity from Valeriana jatamansi. J Nat Prod 2009;72:650-5. [Crossref] [PubMed]
- Jiang HH, Dong FW, Zhou J, et al. Cav2.2 and Cav3.1 calcium channel inhibitors from Valeriana jatamansi Jones. RSC Adv 2017;7:45878-84. [Crossref]
- Bos R, Hendriks H, Scheffer JJC, et al. Cytotoxic potential of valerian constituents and valerian tinctures. Phytomedicine 1998;5:219-25. [Crossref] [PubMed]
- Tao SY, Xiao CR, Wang J, et al. Effects of baldrinal of Valeriana jatamansi on expression of CRF, TPH1 mRNA and 5-HT in rats with irritable bowel syndrome. Zhongguo Zhong Yao Za Zhi 2017;42:347-51. [PubMed]
- Wang R, Chen Y, Huang Q, et al. Chemical constituents from Valeriana jatamansi and their neuroprotective activities. Chinese Traditional Patent Medicine 2017;39:756-60.
- Tan Y, Yang F, Li B, et al. Chemical constituents from Valeriana jatamansi and their antitumor activities. Chinese Traditional Patent Medicine 2019;41:572-6.
- Bounthanh C, Bergmann C, Beck J, et al. Valepotriates, a New Class of Cytotoxic and Antitumor Agents. Planta Medica 1981;41:21-8. [Crossref] [PubMed]
- Wagner H, Jurcic K. On the spasmolytic activity of valeriana extracts. Planta Medica 1979;37:84-6. [Crossref] [PubMed]
- Hazelhoff B, Malingré TM, Meijer DK. Antispasmodic effects of valeriana compounds: an in-vivo and in-vitro study on the guinea-pig ileum. Arch Int Pharmacodyn Ther 1982;257:274-87. [PubMed]
- Li X, Chen T, Lin S, et al. Valeriana jatamansi constituent IVHD-valtrate as a novel therapeutic agent to human ovarian cancer: in vitro and in vivo activities and mechanisms. Curr Cancer Drug Targets 2013;13:472-83. [Crossref] [PubMed]
- Xu J, Li Y, Guo Y, et al. Isolation, Structural Elucidation, and Neuroprotective Effects of Iridoids from Valeriana jatamansi. Biosci Biotechnol Biochem 2012;76:1401-3. [Crossref] [PubMed]
- Xu J, Guo Y, Xie C, et al. Isolation and Neuroprotective Activities of Acylated Iridoids from Valeriana jatamansi. Chem Biodivers 2012;9:1382-8. [Crossref] [PubMed]
- Xu J, Guo X, Guo Y, et al. Iridoids from the roots of Valeriana jatamansi and their biological activities. Nat Prod Res 2012;26:1996-2001. [Crossref] [PubMed]
- Quan LQ, Su LH, Qi SG, et al. Bioactive 3,8-Epoxy Iridoids from Valeriana jatamansi. Chem Biodivers 2019;16:e1800474 [Crossref] [PubMed]
- Yang B, Zhu R, Tian S, et al. Jatamanvaltrate P induces cell cycle arrest, apoptosis and autophagy in human breast cancer cells in vitro and in vivo. Biomed Pharmacother 2017;89:1027-36. [Crossref] [PubMed]
- Lin S, Fu P, Chen T, et al. Minor valepotriates from Valeriana jatamansi and their cytotoxicity against metastatic prostate cancer cells. Planta Med 2015;81:56-61. [PubMed]
- Dong FW, Jiang HH, Yang L, et al. Valepotriates from the Roots and Rhizomes of Valeriana jatamansi Jones as Novel N-Type Calcium Channel Antagonists. Front Pharmacol 2018;9:885. [Crossref] [PubMed]
- Liu YH, Wu PQ, Hu QL, et al. Cytotoxic and antibacterial activities of iridoids and sesquiterpenoids from Valeriana jatamansi. Fitoterapia 2017;123:73-8. [Crossref] [PubMed]
- Wang Y, Shi J, Guo J, et al. Anxiolytic Activity of Valeportiate. Chinese Journal of Experimental Traditional Medical Formulae 2011;17:126-8.
- Vishwakarma S, Goyal R, Gupta V, et al. GABAergic effect of valeric acid from Valeriana wallichii in amelioration of ICV STZ induced dementia in rats. Revista Brasileira de Farmacognosia 2016;26:484-9. [Crossref]
- Sun Y, Lan M, Chen X, et al. Anti-invasion and anti-metastasis effects of Valjatrate E via reduction of matrix metalloproteinases expression and suppression of MAPK/ERK signaling pathway. Biomed Pharmacother 2018;104:817-24. [Crossref] [PubMed]
- Tamura S, Shimizu N, Fujiwara K, et al. Bioisostere of valtrate, anti-HIV principle by inhibition for nuclear export of Rev. Bioorg Med Chem Lett 2010;20:2159-62. [Crossref] [PubMed]
- Glaser J, Schultheis M, Moll H, et al. Antileishmanial and Cytotoxic Compounds from Valeriana wallichii and Identification of a Novel Nepetolactone Derivative. Molecules 2015;20:5740-53. [Crossref] [PubMed]
- Lin S, Shen YH, Zhang ZX, et al. Revision of the Structures of 1,5 Dihydroxy-3,8-epoxyvalechlorine, Volvaltrate B, and Valeriotetrate C from Valeriana jatamansi and V-officinalis. J Nat Prod 2010;73:1723-6. [Crossref] [PubMed]
- Lin S, Chen T, Fu P, et al. Three decomposition products of valepotriates from Valeriana jatamansi and their cytotoxic activity. J Asian Nat Prod Res 2015;17:455-61. [Crossref] [PubMed]
- Yan Z, Zhang T, Xiao T, et al. Anti-anxiety effect of Valeriana jatamansi Jones extract via regulation of the hypothalamus-pituitary-adrenal axis. Neural Regeneration Research 2010;5:1071-5.
- Yu L, Xu KK, Chen CY, et al. A study of the substance dependence effect of the ethanolic extract and iridoid-rich fraction from Valeriana jatamansi Jones in mice. Pharmacognosy Magazine 2015;11:745-9. [Crossref] [PubMed]
- Yan ZY, Zhang TE, Pan L, et al. Action of Valeriana jatamansi Jones on the Apoptosis-related Genes Expression in the Anxiety Model of Rat. Procedia Environ Sci 2011;8:744-50. [Crossref]
- Gilani A, Khan A, Subhan F. Ethnopharmacological basis for the use of Valeriana wallichii in hypermotility disorders of the gut. Iranian Journal of Pharmaceutical Research 2010;3:54.
- Lan M, Zhang Z, Lin Y, et al. Study of Extract of Valeriana Jatamansi Jones Inhibiting Human Colon Cancer SW480 Cells Proliferation and Metastasis. Chinese Archives of Traditional Chinese Medicine 2015;33:293-5.
- Yan Z, Peng J, Qin J, et al. Effect of Valeriana jatamansi Jones on the ethology and GABA, GLY in the brain in the convulsion model of mice. Pharmacology and Clinics of Chinese Materia Medica 2010;26:47-9.
- Yan Z, Zhang T, Peng J, et al. Effect of the extract of Valeriana Jatamansi Jones on the ethology and neurotransmitter in the brain in the anxiety model of rat. Pharmacology and Clinics of Chinese Materia Medica 2008;24:67-9.
- Sajid TM, Rashid S, Ahmad M, et al. Estimation of Cardiac Depressant Activity of Ten Medicinal Plant Extracts from Pakistan. Phytother Res 1996;10:178-80. [Crossref]
- Kour M, Singh H, Kaur J. In vitro anti-oxidant and anti-inflammatory activities of hydroalcoholic extract of leaves of Valeriana Jatamansi. International archives of integrated medicine (IAIM) 2014;1:18-26.
- Joseph L, Rejeesh EP, Narayan RS. Supraadditive effect of hydroethanolic extract of Valeriana wallichii (Indian valerian) root and phenobarbitone against maximal electroshock seizure in mice. Int J Bioassays 2013;2:1158-61.
- Joseph L, Puthallath RE, Rao SN. Acute and chronic toxicity study of Valeriana wallichii rhizome hydro-ethanolic extract in Swiss albino mice. Asian Journal of Medical Sciences 2015;7:49-54. [Crossref]
- Prasad R, Naime M, Routray I, et al. Valeriana jatamansi partially reverses liver cirrhosis and tissue hyperproliferative response in rat. Methods Find Exp Clin Pharmacol 2010;32:713-9. [Crossref] [PubMed]
- He S, Ma X, He R, et al. Intestinal absorption characteristics of 35% ethanol extract from Valeriana jatamansi. Chinese Traditional Patent Medicine 2019;41:485-9.
- Ganta KK, Mandal A, Debnath S, et al. Anti-HCV Activity from Semi-purified Methanolic Root Extracts of Valeriana wallichii. Phytother Res 2017;31:433-40. [Crossref] [PubMed]
- Kalim MD, Bhattacharyya D, Banerjee A, et al. Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement Altern Med 2010;10:77. [Crossref] [PubMed]
- Khuda F, Iqbal Z, Khan A, et al. Anti-inflammatory activity of the topical preparation of Valeriana wallichii and Achyranthes aspera leaves. Pak J Pharm Sci 2013;26:451-4. [PubMed]
- Khuda F, Iqbal Z, Khan A, et al. Screening of selected medicinal plants for their enzyme inhibitory potential–A validation of their ethnopharmacological uses. Pak J Pharm Sci 2014;27:593-6. [PubMed]
- Sridharan S, Mohankumar K, Jeepipalli SP, et al. Neuroprotective effect of Valeriana wallichii rhizome extract against the neurotoxin MPTP in C57BL/6 mice. Neurotoxicology 2015;51:172-83. [Crossref] [PubMed]
- Ghosh S, Debnath S, Hazra S, et al. Valeriana wallichii root extracts and fractions with activity against Leishmania spp. Parasitol Res 2011;108:861-71. [Crossref] [PubMed]
- Subhan F, Karim N, Ibrar M. Anti-inflammatory activity of methanolic and aqueous extracts of Valeriana wallichii DC rhizome. Pakistan Journal of Plant Sciences 2007;13:103-8.
- Sah SP, Mathela CS, Chopra K. Valeriana wallichii DC (Maaliol Chemotype): Antinociceptive Studies on Experimental Animal Models and Possible Mechanism of Action. Pharmacologia 2012;3:432-7. [Crossref]
- Gilani AH, Khan AU, Jabeen Q, et al. Antispasmodic and blood pressure lowering effects of Valeriana wallichii are mediated through K+ channel activation. J Ethnopharmacol 2005;100:347-52. [Crossref] [PubMed]
- Khan AU. Antidiarrhoeal and bronchodilatory potential of Valeriana wallichii. Nat Prod Res 2012;26:1045-9. [Crossref] [PubMed]
- Thusoo S, Gupta S, Sudan R, et al. Antioxidant activity of essential oil and extracts of Valeriana jatamansi roots. Biomed Res Int Biomed Res Int 2014;2014:614187 [PubMed]
- Dua VK, Alam MF, Pandey AC, et al. Insecticidal activity of Valeriana jatamansi (Valerianaceae) against mosquitoes. J Am Mosq Control Assoc 2008;24:315-8. [Crossref] [PubMed]
- Irshad M, Aziz S. GC-MS Analysis and Antimicrobial Activities of Essential oils of Angelica glauca, Plectranthus rugosus, and Valeriana wallichii. Journal of Essential Oil Bearing Plants 2012;15:15-21. [Crossref]
- Feng YX, Wang Y, Geng ZF, et al. Contact toxicity and repellent efficacy of Valerianaceae spp. to three stored-product insects and synergistic interactions between two major compounds camphene and bornyl acetate. Ecotoxicol Environ Saf 2020;190:110106 [Crossref] [PubMed]
- Zhao B, Hao P, Gao A, et al. Study on Antimicrobial and Antioxidant Activities of Essential Oils from Valeriana officinalis L. and Valeriana jatamansi Jones. Nat Prod Res Dev 2013;25:1037-40.
- Sah SP, Mathela CS, Chopra K. Elucidation of possible mechanism of analgesic action of Valeriana wallichii DC chemotype (patchouli alcohol) in experimental animal models. Indian J Exp Biol 2010;48:289-93. [PubMed]
- Agnihotri S, Wakode S, Ali M. Chemical Composition, Antimicrobial and Topical Anti-inflammatory Activity of Valeriana jatamansi Jones. Essential Oil. Journal of Essential Oil Bearing Plants 2011;14:417-22. [Crossref]
- Syed SN, Rizvi W, Kumar A, et al. A study to evaluate antioxidant and hepatoprotective activity of aqueous extract of roots of Valeriana wallichii in CCl4 induced hepatotoxicity in rats. Int J Basic Clin Pharmacol 2017;3:354-8. [Crossref]
- Sahu S, Ray K, Yogendra Kumar MS, et al. Valeriana wallichii root extract improves sleep quality and modulates brain monoamine level in rats. Phytomedicine 2012;19:924-9. [Crossref] [PubMed]
- Katoch O, Kaushik S, Kumar MSY, et al. Radioprotective property of an aqueous extract from Valeriana Wallichii. J Pharm Bioallied Sci 2012;4:327-32. [Crossref] [PubMed]
- Rehni AK, Pantlya HS, Shri R, et al. Effect of Chlorophyll and aqueous extracts of Bacopa monniera and Valeriana wallichii on ischemia and reperfusion induced cerebral injury in mice. Indian J Exp Biol 2007;45:764-9. [PubMed]
- Sharma P, Kirar V, Meena DK, et al. Adaptogenic activity of Valeriana wallichii using cold, hypoxia and restraint multiple stress animal model. Biomed Aging Pathol 2012;2:198-205. [Crossref]
- Toolika E, Bhat NP, Shetty SK. A comparative clinical study on the effect of Tagara (Valeriana wallichii DC.) and Jatamansi (Nardostachys jatamansi DC.) in the management of Anidra (primary insomnia). Ayu 2015;36:46-9. [Crossref] [PubMed]
- Mao X, Wang J. J W. Main Pharmacodynamic Study on Valeriana Wallichii dc. Journal of Yunnan University of Traditional Chinese Medicine 2008;31:34-7.
- Zhang XM, Zhu JL, Sun Y, et al. Anxiolytic potency of iridoid fraction extracted from Valeriana jatamansi Jones and its mechanism: a preliminary study. Nat Prod Res 2018;32:2071-5. [Crossref] [PubMed]
- Zhu J, Xu K, Zhang X, et al. Studies on the regulation of lipid metabolism and its mechanism of the iridoids rich fraction in Valeriana jatamansi Jones. Biomed Pharmacother 2016;84:1891-8. [Crossref] [PubMed]
- Xu K, Lin Y, Zhang R, et al. Evaluation of safety of iridoids rich fraction from Valeriana jatamansi Jones: Acute and sub-chronic toxicity study in mice and rats. J Ethnopharmacol 2015;172:386-94. [Crossref] [PubMed]
- Gao H, Tan Y. Study on the pharmacology from two extracts of Valeriana jatamansi. West China Journal of Pharmaceutical Sciences 2014;29:154-7.
- Zhang T, Chen C, Chen C, et al. Effect on TGF-beta Signaling Pathway of the Total Flavonoids from Valeriana jatamansi Jones in Hepatocarcinoma 22-bearing Mice. 2012 International Conference on Biomedical Engineering and Biotechnology. IEEE, 2012:210-2.
- Zhai X, Kong Z, Wang S, et al. Study on anti-anxiety activity of extract and total valepotriate in Valerianae Rhizoma. Chin Tradit Herb Drugs 2016;47:1361-5.
- Wagner H, Jurcic K, Schaette R. Comparative studies on the sedative action of Valeriana extracts, valepotriates and their degradation products (author's transl). Planta Medica 1980;39:358-65. [Crossref] [PubMed]
- Sah SP, Mathela CS, Chopra K. Antidepressant effect of Valeriana wallichii patchouli alcohol chemotype in mice: Behavioural and biochemical evidence. J Ethnopharmacol 2011;135:197-200. [Crossref] [PubMed]
- Wang J, Shi RR, Wang J, et al. Effects of iridoids constituents of Valeriana jatamansi Jones ZXX on gastrointestinal sensitivity and gastrointestinal hormones in rats with irritable bowel syndrome. Journal of Changchun University of Traditional Chinese Medicine 2014;30:396-9.
- Huang Y, Pan Y, Zhang J, et al. Determination and Analysis of Oligosaccharides in Valeriana Jatamansi Jones and 5 Drugs for Gastrointestinal Diseases. Journal of Kunming Medical University 2012;33:9-13.
- Wasowski C, Marder M, Viola H, et al. Isolation and Identification of 6-Methylapigenin, a Competitive Ligand for the Brain GABA A Receptors, from Valeriana wallichii. Planta Medica 2002;68:934-6. [Crossref] [PubMed]
- Mathela CS, Chanotiya CS, Sammal SS, et al. Compositional diversity of terpenoids in the Himalayan Valeriana genera. Chem Biodivers 2005;2:1174-82. [Crossref] [PubMed]
- Bos Rein, Woerdenbag Herman J, Hendriks Henk, et al. Composition of the Essential Oil from Roots and Rhizomes of Valeriana wallichii DC. Flavour Frag J 1997;12:123-31. [Crossref]
- Verma RS, Verma RK, Padalia RC, et al. Chemical diversity in the essential oil of Indian Valerian (Valeriana jatamansi Jones). Chem Biodivers 2011;8:1921-9. [Crossref] [PubMed]
- Zhang M, Zhao M, Yin C, et al. GC-TOFMS analysis of volatile oil extraction form Valeriana jatamansi Jones by three different methods. China Journal of Traditional Chinese Medicine and Pharmacy 2016;31:3312-7.
- Bhatt ID, Rawat S, Rawal RS. Antioxidants from medicinal plants Biotechnology for Medicinal Plants. Springer Berlin Heidelberg, 2013:295-326.
- Park IK. Fumigant Toxicity of Oriental Sweetgum (Liquidambar orientalis) and Valerian (Valeriana wallichii) Essential Oils and Their Components, Including Their Acetylcholinesterase Inhibitory Activity, against Japanese Termites (Reticulitermes speratus). Molecules 2014;19:12547-58. [Crossref] [PubMed]
- Lv Y, Liu J, Shi S, et al. Anxiolytic effect of antianxietic compound prescription capsule on acute stress in rats and influence upon expression of ERK/CREB signaling pathway and BDNF in the brain of rats. Chinese Pharmacological Bulletin 2015;31:1614-9.
- Peng M, Shi J, Zheng H, et al. Anxiolytic-like effects of Antianxietic Compound Prescription with Valerianae Jatamansi Rhizoma et Radix. Chinese Traditional and Herbal Drugs 2011;42:2283-6.
- You JS, Peng M, Shi JL, et al. Evaluation of anxiolytic activity of compound Valeriana jatamansi Jones in mice. BMC Complement Altern Med 2012;12:223. [Crossref] [PubMed]
- Zhu Z, Shen W, Tian S, et al. F3, a novel active fraction of Valeriana jatamansi Jones induces cell death via DNA damage in human breast cancer cells. Phytomedicine 2019;57:245-54. [Crossref] [PubMed]
- Li J, Lin L. Anti-Viral Activity of Qiuxieling and Erxieting in Rotavirus SA11-Infected MA104 Cells. Letters in Biotechnology 2016;27:675-8.
- Yan X, Hong Y, Shi J, et al. Influence of iridoid from Valeriana jatamansi on 5-HT and 5-HIAA in rats with irritable bowel syndrome. China Journal of Chinese Materia Medica 2011;36:1235-8. [PubMed]
- Zhang P, Chen X, Wang Y, et al. Action Mechanism of Valeriana Jatamansi Jones in Treating Rotavirus Enteritis in Infants. Chinese General Practice 2010;13:610-2.
- Murakami N, Ye Y, Kawanishi M, et al. New Rev-Transport Inhibitor with anti-HIV Activity from Valerianae Radix. Bioorg Med Chem Lett 2002;12:2807-10. [Crossref] [PubMed]
- Khuda F, Iqbal Z, Khan A, et al. Antimicrobial and anti-inflammatory activities of leaf extract of Valeriana wallichii DC. Pak J Pharm Sci 2012;25:715-9. [PubMed]
- Lei Y, Yu J, Liu S, et al. Study on the antibacterial effect of Miao Nationality Medicine compound wo-lai-liu solution. Chinese Journal of Ethnomedicine and Ethnopharmacy 2016;25:21-2.
- Wang P, Meng X, Gao X, et al. Study on the External Bacteriostasis of Three Chinese Herbal Medicine. Journal of Guiyang Medical College 2014;39:508-10.
- Ma L, Li P, Song Y, et al. Effects of Drug Serum of Valeriana jatamansi on the Splenic Lymphocytes and Peritoneal Macrophages in Mice. Medicinal Plant 2010;1:55-7.
- Priyambada S, Jagadeesh P, Mayuri T, et al. Dose Dependent Anti-Inflammatory Effect of Valeriana Wallichii in 0.1 Ml of 1% Carrageenan Induced Hind Paw Edema in Male Albino Rats. IOSR-JPBS 2015;10.
- Tian S, Wang Z, Wu Z, et al. Valtrate from Valeriana jatamansi Jones induces apoptosis and inhibits migration of human breast cancer cells in vitro. Nat Prod Res 2020;34:2660-3. [Crossref] [PubMed]
- Quan LQ, Liu H, Li RT, et al. Three new 3,8-epoxy iridoids from the roots and rhizomes of Valeriana jatamansi. Phytochem Lett 2019;34:35-8. [Crossref]
- Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct 2008;213:63-72. [Crossref] [PubMed]
Cite this article as: Ma Y, Pei S, He N, Lai Q, Zhuang M, Bian Z, Lin C. A narrative review of botanical characteristics, phytochemistry and pharmacology of Valeriana jatamansi jones. Longhua Chin Med 2021;4:5.

