
Effect of Moshen Digui prescription for treatment of idiopathic membranous nephropathy—protocol for a randomized, controlled, and single-blind trial
Introduction
Membranous nephropathy (MN) has been the leading cause of the nephrotic syndrome in nondiabetic adults (1-3). MN can be induced by many secondary causes, such as drugs, infections, and underlying diseases (4). However, most patients of MN are idiopathic (5). IMN is a kind of antibody-mediated autoimmune disease, which can be diagnosed by renal biopsy. The characterized renal biopsy of IMN is deposition of immune complex along the glomerular basement membrane. Moreover, IMN patients has high risk for progressive deterioration of renal function. Research found that 30–40% IMN patients could develop into ESRD (6).
As we know, ACEI/ARB is the basic therapy for IMN. Research showed that ACEI/ARB has beneficial effect on decreasing proteinuria for IMN patients with non-nephrotic-range proteinuria (7,8). When patients, proteinuria ≥4 g/24 h after 6-month conservative therapy, they should be applied immunosuppressive therapy (9). Cyclosporine and tacrolimus, mycophenolate mofetil (MMF), rituximab, and adrenocorticotropic hormone (ACTH) are recommended for IMN patients for immunosuppressive therapies (10). However the immunosuppressive therapies have side effects such as infection, tumor, and so on. Moreover, there are many IMN patients has high level of proteinuria and high risk of progression to ESRD after these active treatments. Thus, faced with the limitations of currently available treatments, searching additional interventions for IMN remains worthy of exploration.
Traditional Chinese medicine (TCM) has been widely used in the treatment of IMN (11). TCM showed beneficial effect for IMN patients in our clinical practice. In recent years, clinical trial has demonstrated the effectiveness and safety of TCM in the treatment of IMN (12). The TCM formula used in our trial is Moshen Digui (MSDG). As a kind of empirical formula, MSDG has been widely used in the treatment of IMN. The efficacy and safety of MSDG has been demonstrated by summarizing of clinical data of many IMN patients. However, there is no high-quality randomized, controlled trial for MSDG in the treatment of IMN. In our study, placebo will be used for comparison. A randomized, controlled, and single-blind Trial will be performed in our study. Our study will provide evidence for MSDG in the treatment of IMN.
We present the following article in accordance with the SPIRIT reporting checklist (available at http://dx.doi.org/10.21037/lcm-20-10).
Methods
Ethics, consent, and permissions
Our trial has been approved by the ethics committee of Beijing Traditional Chinese medicine Hospital (Approval No. 2017BL-049-02). It has been registered on the Chinese Clinical Trial Registry under number ChiCTR1800015866. The trial will be performed in accordance with the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) guidelines (13). All participants will be informed relative content about our trial including the purpose of the trial and the possible risks and benefits. All visits will be documented in Case Report Forms (CRFs). Data are stored in a research database in accordance with the Standard Protection Authority.
Study design and setting
Our study is a randomized, controlled, and single-blind trial. The flowchart of our trial is shown in Figure 1. Sample size of our study was calculated by computational formula. The computational formula is as follows:
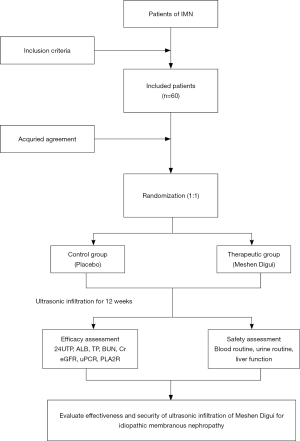
N= [(Zα+Zβ) σ/δ] 2(Q1-1+Q2-1). The parameters are as follows: α=0.05, β=0.20, Q1=Q2=0.5. A total of 60 IMN patients will be randomized into two groups at a ratio of 1:1. The two groups are “control group” and “therapeutic group” respectively. All patients will be recruited from Beijing Traditional Chinese Medicine Hospital. If a patient consents, patients’ information will be collected and biochemical test results will be detected at the first visit. Eligible participants will be randomly divided into different groups and will be received different treatment. To explore efficacy and safety of MSDG, biochemical test will be detected every four weeks regularly. The timing of treatment visits and data collection is detailed in Table 1.
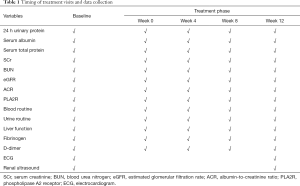
Full table
Randomization and blinding
Complete randomization will be performed in our trial. A randomization sequence 1 to 60 will be generated by SAS8.2 software. The serial number are printed on treatment cards. Patients will be received treatment cards and randomly divided into different groups according to their sequence of diagnosis and treatment. The random allocation sequence will be produced by an independent researcher who is not involved in the recruitment, evaluation and intervention of participants. Before intervention commencement, the research staff will inform eligible participants of their intervention allocation by telephone. Patients of odd number will be taken into therapeutic group. Patients of even number will be taken into control group. All processes will be performed by an independent researcher.
Our trial will use single-blind method. Patients will not know their real therapeutic method. However, all information is open for researchers. Placebo of MSDG will be used for control. The paster of placebo will be made as the same smell, size, colour, and appearance with the paster of MSDG.
Participants
Participants recruitment
Advertisements of our trial will be showed on hospital bulletin boards in Beijing Traditional Chinese Medicine Hospital, capital medical university. Patients who show interest in participating can communicate with relative researchers. The information of our trial will be provided to patients. Once patients agree to participate in our trial, they will be assessed by their demographic data and information about medical history, concomitant medication, physical examination, vital signs, and regular test results. All participants should conform to the diagnostic criteria of IMN. Diagnostic criteria for IMN are based on the criteria set by KDIGO Clinical Practice Guideline (14). TCM syndrome type of all participants should be deficiency of spleen and kidney with blood stasis. The diagnostic standards of deficiency of spleen and kidney with blood stasis include primary symptoms and secondary symptoms. Primary symptoms include Lumbago, edema, weakness, gloomy complexion, and dyspepsia. Secondary symptoms include limb numbness, scaly dry skin, frequent micturition at night, and diarrhea. Moreover, eligible patients should satisfy the inclusion and exclusion criteria.
Inclusion criteria
- Conform to the diagnostic criteria of IMN;
- 24-hour urinary protein quantification is less than 4g and renal function is normal;
- There are no contraindications of ACEI/ARB, such as renal artery stenosis, hyperkalemia, and isolated kidney;
- Patients who aged 18 to 75.
Exclusion criteria
- Patients who has applied hormone, immunosuppressant, and/or tripterygium Glycosides in the past six months;
- Patients of acute renal failure;
- Patients who has high blood pressure and infection and has not been effectively controlled;
- Patients who has severe primary disease, malignant tumor, and mental disease;
- Patients who has allergic constitution and has history of drug allergy;
- Patients who has local skin lesion at the waist;
- Patients who is pregnant and lactating women.
Elimination criteria
- Patients who are misdiagnosed;
- Patients who are not regularly follow-up recorded and laboratory report is incomplete;
- Patients who have applied hormone, immunosuppressant during the study period.
Termination criteria
- Patients whose Scr was significantly increased (≥30%);
- Patients who have severe complications and infection during the research.
Interventions
Basic treatment
All participants should be received high quality low protein diet (1 g/kg/d). All participants should have adequate time to rest. Lipid-lowering therapy is recommended for all participants and anticoagulation therapy is recommended for participants who have hypercoagulable state.
Intervention method
Participants of two groups are given one capsule of irbesartan (150 mg per grain) once daily for 12 weeks. Participants of control group are received ultrasonic infiltration of placebo. Participants of therapeutic group are received ultrasonic infiltration of MSDG. Treatment of ultrasonic infiltration are performed once every other day. Ultrasonic infiltrations are lasted 30 min at a time for 12 weeks. When participants’ blood pressure<90/60 mmHg, the irbesartan will be decrement. The participant cannot use hormone, immunosuppressant, and/or tripterygium Glycosides during the trial.
Irbesartan capsules (150 mg per capsules) will be provided by Sanofi Pharmaceutical Company (Hangzhou, China). MSDG consist of elven herbs: Chinese Foxglove, Carapax Testudinis, Astragalus membranaceus, Codonopsis pilosula, Atractylodes macrocephala Koidz, Angelica sinensis, Paeonia lactiflora Pall, Ligusticum chuanxiong Hort, Alisma plantago-aquatica Linn, Rubus idaeus, and Hedyotis diffusa. MSDG and placebo will be made as paster. The process as follows: MSDG and placebo will be smashed and extracted by supercritical CO2 fluid extraction. After that, they will be low temperature concentrated and gelatinized. The paster of placebo will be made as the same smell, size, colour, and appearance with the paster of MSDG.
Outcome measurements
Primary outcome
In our study, 24-h urinary protein is detected for the primary measurements. Urinary protein of 24-h is detected at 0, 4, 8, 12 weeks respectively. Clinical therapeutic effect is evaluated according to the change of 24-h urinary protein. Therapeutic effect consists of three grades.
- Complete remission: 24-h urinary protein <0.3 g or urinary protein/creatinine <300 mg/g; normal renal function; serum albumin >35 g/L; urinary protein is negative.
- Partial remission: 24 h urinary protein among 0.3 to 3.5 g; or urinary protein/creatinine 300–3,500 mg/g; or 24 h urinary protein decreases 50% compared with base line; renal function is stable (serum creatinine increases less than 20% compared with baseline).
- Non-remission: 24 h urinary protein >3.5 g and decreases less than 50% compared with baseline.
Secondary outcomes
Secondary outcomes consist of serum albumin, serum total protein, serum creatinine, serum blood urea nitrogen, eGFR, urinary albumin/creatinine, and PLA2R. They are also detected at 0, 4, 8, 12 weeks.
Safety assessment
Blood routine, urine routine, liver function, fibrinogen, and D-dimer are detected at 0, 4, 8, 12 weeks. ECG and renal ultrasound are detected at before and after treatment. Local skin is observed to confirm no ecchymosis, rash, and breakage at every treatment. Any adverse events will be recorded and assessed. Participants who suffer harm from trial will obtain free treatment and compensation.
Investigator training and quality control
To guarantee the quality of our trial, all investigator will receive the courses of theory and practice. Our courses will include how to screen the eligible participants, use the random envelope, and perform the process of ultrasonic infiltration. All investigators will take a test at the end of courses. When they pass the test, they will obtain a certificate. Moreover, specially trained medical officers will perform an inspection throughout our trial. Moreover, the data monitoring committee (DMC) will be established in our trial. The DMC has no competing interests and is independent from the sponsor.
Data collection
The tools of data collection will be created for each patient. The pages are colour coded by time point, and preceded by a log page to complete the interview dates and a front cover with the patient’s ID number. Data of baseline will be collected at the beginning of our trial. The data of all patients will be collected at 4, 8, 12 weeks respectively. To reduce follow-up attrition, we will call to participants to remind them of the follow up data collection appointment.
Data management
Data will be collected by trained researchers. All data will be entered into predesigned Excel spreadsheets by two researchers. This will be exported to IBM-SPSS-22 for analysis, including creation of graphs, descriptive statistics, univariate analysis and multiple regression.
Statistical analysis
Data will be analyzed by statistical SPSS 20.0 software in our study. Data will be presented as mean ± SEM for continuous data. Statistical analyses will be performed by Student’s 𝑡-test for comparison of 2 groups (data can be analyzed by parametric test). If data can’t be analyzed by parametric test, rank-sum test will be used for statistical analyses. Statistical analyses will be performed by Chi-squared test for categorical data. For ranked data, rank-sum test will be used for statistical analyses. P<0.05 will be considered statistically significance and P<0.01 will be considered highly statistics significance.
Discussion
IMN, as a kind of common disease, has been the main cause of the nephrotic syndrome in nondiabetic adults (1). Meanwhile, IMN is a refractory disease. Although many active treatments have been applied for IMN patients, there are many patients developed into ESRD (6,15). TCM has been considered as a basic or complementary method in the treatment of IMN in China (11). More importantly, TCM compound exhibits its efficacy and safety in the treatment of IMN in clinical practice (16).
MSDG, as a kind of TCM compound, has been widely used in the treatment of IMN in our clinical practice. We found that MSDG can significantly reduce proteinuria and delay progression of IMN. However, there is no high-quality randomized, controlled trial for MSDG in the treatment of IMN. Therefore, our clinical trial will be performed. To guarantee the quality, every procedure will be under strict quality control. Complete randomization will be performed in our trial. Meanwhile, placebo will be used for control in our trial. The paster of placebo will be made as the same smell, size, colour, and appearance with the paster of MSDG. The 24-h urinary protein, as the key outcome, will be detected every 4 weeks.
The method of administration in our trial is ultrasonic infiltration. As we know, taking medicine orally is the traditional drug-delivery way. However, the poor taste of TCM can reduce patients’ compliance. Ultrasonic infiltration is a new kind of drug-delivery way, which has been approved by FDA. Previous studies have demonstrated that the efficacy and safety in the treatment of many diseases (17-20). For patients received a long-term TCM treatment, ultrasonic infiltration may be a kind of effective method of administration. Thus, ultrasonic infiltration will be used for the treatment of IMN in our trial.
Our study will provide the effect of ultrasonic infiltration of MSDG prescription for IMN patients.
Acknowledgments
Funding: This work was supported by Application of clinical features of capital city of science and Technology Commission China BEIJING Special subject (No. Z171100001017032); Cultivation Program of Beijing City Hospital (No. pZ2018012).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Bo Li and Lyubima Despotova-Toleva) for the series “Narrative & Evidence-based Medicine for Traditional Medicine: from basic research to clinical practice and trail” published in Longhua Chinese Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SPIRIT reporting checklist. Available at http://dx.doi.org/10.21037/lcm-20-10
Peer Review File: Available at http://dx.doi.org/10.21037/lcm-20-10
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-20-10). The series “Narrative & Evidence-based Medicine for Traditional Medicine: from basic research to clinical practice and trail” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. It has been registered on the Chinese Clinical Trial Registry under number ChiCTR1800015866. All participants will be informed relative content about our trial including the purpose of the trial and the possible risks and benefits.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seitz-Polski B, Lambeau G, Esnault V. Membranous nephropathy: Pathophysiology and natural history. Nephrol Ther 2017;13:S75-S81. [Crossref] [PubMed]
- Königshausen E, Sellin L. Recent Treatment Advances and New Trials in Adult Nephrotic Syndrome. Biomed Res Int 2017;2017:7689254 [Crossref] [PubMed]
- Alfaadhel T, Cattran D. Management of Membranous Nephropathy in Western Countries. Kidney Dis (Basel) 2015;1:126-37. [Crossref] [PubMed]
- Xu NX, Xie QH, Sun ZX, et al. Renal Phospholipase A2 Receptor and the Clinical Features of Idiopathic Membranous Nephropathy. Chin Med J (Engl) 2017;130:892-8. [Crossref] [PubMed]
- Hofstra JM, Fervenza FC, Wetzels JF. Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol 2013;9:443-58. [Crossref] [PubMed]
- Hofstra JM, Wetzels JF. Introduction of a cyclophosphamide-based treatment strategy and the risk of ESRD in patients with idiopathic membranous nephropathy: a nationwide survey in the Netherlands. Nephrol Dial Transplant 2008;23:3534-8. [Crossref] [PubMed]
- Polanco N, Gutiérrez E, Covarsí A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 2010;21:697. [Crossref] [PubMed]
- Tran TH, J, Hughes G, Greenfeld C, et al. Overview of current and alternative therapies for idiopathic membranous nephropathy. Pharmacotherapy 2015;35:396-411. [Crossref] [PubMed]
- du Buf-Vereijken PW, Branten AJ, Wetzels JF. Idiopathic membranous nephropathy: outline and rationale of a treatment strategy. Am J Kidney Dis 2005;46:1012-29. [Crossref] [PubMed]
- Cattran D, Brenchley P. Membranous nephropathy: thinking through the therapeutic options. Nephrol Dial Transplant 2017;32:i22-i29. [Crossref] [PubMed]
- Xu J, Hu X, Xie J, et al. Management of Membranous Nephropathy in Asia. Kidney Dis (Basel) 2015;1:119-25. [Crossref] [PubMed]
- Chen Y, Deng Y, Ni Z, et al. Efficacy and safety of traditional chinese medicine (Shenqi particle) for patients with idiopathic membranous nephropathy: a multicenter randomized controlled clinical trial. Am J Kidney Dis 2013;62:1068-76. [Crossref] [PubMed]
- Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586 [Crossref] [PubMed]
- Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Inter 2012;2:139-274.
- Couser WG. Primary Membranous Nephropathy. Clin J Am Soc Nephrol 2017;12:983-97. [Crossref] [PubMed]
- Shi B, Zhang RR, Liang Y, et al. Efficacy of Traditional Chinese Medicine Regimen Jian Pi Qu Shi Formula for Refractory Patients with Idiopathic Membranous Nephropathy: A Retrospective Case-Series Study. Evid Based Complement Alternat Med 2018;2018:5854710 [Crossref] [PubMed]
- Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol 2000;9:165-9. [Crossref] [PubMed]
- Tachibana K, Tachibana S. Transdermal delivery of insulin by ultrasonic vibration. J Pharm Pharmacol 1991;43:270-1. [Crossref] [PubMed]
- Tezel A, Paliwal S, Shen Z, Mitragotri S. Low-frequency ultrasound as a transcutaneous immunization adjuvant. Vaccine 2005;23:3800-7. [Crossref] [PubMed]
- Mitragotri S, Blankschtein D, Langer R. Transdermal drug delivery using low-frequency sonophoresis. Pharm Res 1996;13:411-20. [Crossref] [PubMed]
Cite this article as: Cui F, Sun X, Wang Y, Cai Z, Meng Y, Zhao W. Effect of Moshen Digui prescription for treatment of idiopathic membranous nephropathy—protocol for a randomized, controlled, and single-blind trial. Longhua Chin Med 2021;4:8.

