
Bioactivity of hydro-alcoholic extract of Petroselinum crispum
Introduction
Plants have been and still are a source of medicinal agents, and, thanks to their use in traditional medicine, a high number of modern drugs have been obtained from natural sources (1).
Recently, much attention has been paid to extracts and biologically active compounds isolated from plant species used extensively in folk medicine to treat ailments and diseases, in order to analyse their pharmacological activities and to better understand what their applications can be (2-5).
Petroselinum crispum (P. crispum), belonging to the family Apiaceae, is a plant widely cultivated in the south-eastern Europe and western Asia (6). It is commonly called “parsley” and appreciated for its medicinal properties long before became used as food or spice (7). In fact, in addition to its culinary use, P. crispum is used for different medicinal purposes in traditional medicine of different countries. The leaves have been employed as antitussive and diuretic, for the treatment of kidney stones, hemorrhoids, gastrointestinal disorders, hypertension, diabetes, amenorrhoea, dysmenorrhea and dermatitis (8). The seeds and roots of the plant have been used to treat numerous digestive problems, including diarrhoea, ulcer and cholic pain (9). P. crispum essential oils and extracts demonstrate antioxidant, anti-inflammatory, calcium channel-blocking (in the intestine and uterus muscle), cancer preventive, and laxative properties (10-13). However, P. crispum essential oils can also be phototoxic and abortifacient and should never be consumed or applied to the skin without caution (6). The methanol extracts of P. crispum express good antimicrobial activity against many bacteric strains such as B. subtilis, P. aeruginosa, S. epidermidis, S. aureus, E. coli, Klebsiella pneumoniae, and Saccharomyces cerevisiae, while hydro-alcoholic extract of the same plant demonstrates a significant inhibition of P. aeruginosa (14). The aqueous extracts of parsley show bactericidal effects against Helicobacter pylori and inhibit adhesion of the bacteria to animal stomach section (15).
The bioactivity and the medical use of parsley are mostly due to its phytochemical composition, rich in many bioactive components such as flavonol glycosides of quercetin, apiol, myristicin, luteolin, terpenes, phthalates, furanocoumarins, apiin, carotenoids, ascorbic acid and tocopherol (16). Previous investigations on P. crispum are mostly focused on its antioxidant properties which have been attributed mainly to their polyphenol contents as it is well demonstrated that plants containing high-level of polyphenols have a great importance as natural antioxidants (17). Cultivar, geographical location and growth conditions of the plant, together with extraction method used, may influence the polyphenolic content of the extracts and, consequently, their bioactivity (18-21). For these reasons, chemical analysis of each plant extract should be performed in order to correlate total amount of polyphenols present with its bioactivity, so to focus the use of the extract in specific applications.
This study aimed to quantify the total phenolic content of an ethanolic extract obtained from P. crispum leaves and to determine its in vitro antioxidant activity, both in non-cellular and cellular systems. Moreover, cytotoxic effect towards NIH3T3 mouse fibroblasts and human breast adenocarcinoma cell line MDA-MB231, mutagenicity and interference with human blood coagulation factor, were also evaluated in order to correlate the phenolic content of the extract with its wide range of in vitro bioactivity and to understand what its applications can be.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/lcm-20-47).
Methods
Thrombin, Dulbecco’s Modified Eagle’s Medium (DMEM), trypsin solution, and all the solvents used for cell culture as well as Fast Blue BB (FBBB) [4-benzoylamino-2,5-dimethoxybenzenediazonium chloride hemi-(zinc chloride)] salt, gallic acid and all the other reagents were purchased from Sigma-Aldrich (Germany). Mouse immortalized fibroblasts NIH3T3 and human breast adenocarcinoma cells MDA-MB-231 were from American Type Culture Collection (USA). Ames test kit was supplied from Xenometrix (Switzerland). Human poor platelet plasma (PPP) was supplied form CliniSciences (Italy).
P. crispum extract preparation
P. crispum fresh leaves were collected in Val d’Orcia (42°56′02″N 11°38′17″E Tuscany, Italy). The fresh leaves were washed three times in distilled water and left to dry at room temperature for 24 hours. Then, the leaves were chopped with a scalpel. The extract was obtained by putting 20 g of chopped leaves in 80 g of 60% (v/v) ethanol (EtOH) for 48 hours at room temperature in a shaker incubator. At the end of the incubation, the suspension was filtered by a 0.45 µm Whatman membrane filter, and dried using a rotary evaporator. The dry extract obtained was weighted and the percentage yield was expressed as air-dried weight of plant material. Samples then were stored at 2–4 °C until it was time to conduct further analysis.
FBBB total phenolic assay
The FBBB procedure (22,23) was utilized to quantify total phenolic content of Pcex. First of all, gallic acid calibration standards with concentrations of 0, 10, 25, 50, 100, 250 and 500 µg/mL in 60% EtOH were prepared. Then, 1 mL of each working standard solution was transferred to borosilicate tubes and 0.1 mL of 0.1% FBBB reagent was added to all gallic acid standard solutions. All the samples were mixed for 1 min and successively 0.1 mL of 5% NaOH was added. The solutions were incubated at room temperature, in the dark, for 60 min. At the end of the incubation, 1 mL of each standard concentration solution was transferred in PMMA semi-micro disposable cuvettes and the optical density, was measured at 420 nm. The gallic acid concentrations were plotted against the optical density. The regression equation was y=0.006x–0.0299 and the coefficient of correlation R2=0.9983. The total phenolic content of the sample was expressed as mg gallic acid equivalents (GAEs), in 1 g of dry sample.
Hydrogen peroxide scavenging assay
The ability of Pcex to scavenge hydrogen peroxide was estimated according the method of Ruch et al. (24). A solution of H2O2 (2 mM) was prepared in phosphate buffer (50 mM, pH=7.4). Aliquots (0.05, 0.1, 0.2, 0.3 and 0.4 mL) of Pcex at a concentration of 24 mg/mL, were transferred into test tubes and their volumes were made up to 0.4 mL with 50 mM phosphate buffer at pH=7.4. After adding 0.6 mL of hydrogen peroxide solution, tubes were vortexed and the absorbance of H2O2 at 230 nm was determined after 10 min of incubation, against a blank containing phosphate buffer and EtOH 60% without H2O2. The percentage of hydrogen peroxide scavenging was calculated as follows:
% scavenged H2O2 = [(Ai – At)/Ai] × 100
Where Ai is the absorbance of control and At is the absorbance of test samples.
Antiradical capacity: DPPH assay
Scavenger capacity of Pcex towards DPPH radical was calculated and its antioxidant activity reported in GAE through EPR spectroscopy.
Continuous-wave X-band (CW, 9 GHz) EPR spectra were recorded using a Bruker E580 ELEXSYS Series spectrometer (Bruker, Italy), with the ER4122SHQE cavity. EPR measurements were performed filling 1.0 mm ID × 1.2 mm OD quartz capillaries placing them into a 3.0 mm ID × 4.0 mm OD suprasil tube.
A stock solution of DPPH was prepared (0.2 mM in ethanol) and the final concentration of the radical in each sample was 0.16 mM. The extract was added with one-fourth volume in respect to that of the radical. The addition of the extract was taken from the stock solution and it was incubated for 15 minutes.
The area of the EPR spectra was calculated by the double integral of the DPPH signal and the scavenger percentage was calculated using the Eq. [2] where A0 is the area of the DPPH signal without the addition of the extract, Aextract is the area the DPPH signal after the addition of the extract.
The scavenger percentage of the EPR signal after addition of the antioxidant to the DPPH radical solution was calculated as follow:
scavenger % = (A0 – Aextract)/A0 × 100
GAE antioxidant activity through DPPH assay
A stock solution of DPPH radical 1 mM in EtOH was freshly prepared and used within 5 hours. A stock solution of gallic acid 0.2 mM in EtOH was prepared. The calibration curves were built using a standard solution of gallic acid. To a fixed volume of the DPPH solution (100 µL) was added the gallic acid standard solution with different linear increasing volumes (10–100 µL) and a known volume (100 µL) of the extract. After 15 minutes of incubation in the dark at room temperature, the EPR spectra were recorded. The antioxidant activity was plotted through the decay percentage of the area of the DPPH signal versus increasing concentrations of gallic acid standard solution. The area of the EPR spectra was calculated through the double integral of the DPPH signal.
The decay percentage for the plotting refers to the Eq. [3] where A0 is the area of the DPPH signal without the addition of the antioxidant or extract, AS is the area the DPPH signal after the addition of scavenger agent such as antioxidant gallic acid or the extract.
The decay area percentage expressed in GAE was obtained through the calibration curve built with the standard solution (R2=0.928) and reporting the area of the DPPH EPR signal after the addition of the extract. The measurements were repeated in triplicate and the antioxidant activity of the extract was expressed as mole/g of acid gallic equivalent (25).
decay % = (A0 – AS)/A0 × 100
Cytotoxicity
In order to evaluate the in vitro cytotoxicity of new products, the direct contact tests was used (26). This test is suitable for sample with various shapes, sizes or physical status (i.e., liquid or solid). The evaluation of in vitro acute toxicity does not depend on the final use for which the product is intended, and the document ISO 10995-5:2009 recommends many cell lines from American Type Collection. Among them, to test Pcex cytotoxicity, NIH3T3 mouse fibroblasts were chosen (27). Moreover, in order to evaluate the cytotoxicity activity of Pcex towards tumoral cells, the same test was repeated by using human breast adenocarcinoma cells MDA-MB-231.
Both NIH3T3 and MDA-MB-231 cells were propagated in DMEM supplemented with 10% fetal calf serum, 1% L-glutamine-penicillin-streptomycin solution, and 1% MEM non-essential amino acid solution, and incubated at 37 °C in a humidified atmosphere containing 5% CO2. Once at confluence, the cells were washed with PBS 0.1 M, separated with trypsin-EDTA solution and centrifuged at 1,000 rpm for 5 minutes. The pellet was re-suspended in complete medium (dilution 1:15). Cells (1.5×104) suspended in 1 mL of complete medium were seeded in each well of a 24 well round multidish and incubated at 37 °C in an atmosphere of 5% CO2. Once reached the 50% of confluence (i.e., after 24 hours of culture), the culture medium was discharged and the test compounds, properly diluted in completed medium, were added to each well. All samples were set up in six replicates. Complete medium was used as negative control. After 24 hours of incubation, cell viability was evaluated by neutral red uptake (NRU) assay following the procedure previously reported (28,29).
Hydrogen peroxide treatment of cells
To determine the protective effect of alcoholic extract of Pcex against oxidative stress, NIH3T3 and MDA-MB-231 cells were pre-incubated with different concentrations of hydrogen peroxide (0.1, 0.2, 0.3, 0.9, 1.0, 1.1, 1.3, 1.5, 1.6 µM) for 15 min, than washed and incubated for 24 hrs with 0.3% v/v Pcex at a concentration of 1.2 mg/mL. Cell viability was evaluated after 24 hrs of incubation at 37 °C in 5% CO2 by NRU assay (28,29).
Mutagenicity assay: Ames test
The TA100 and TA98 strains of Salmonella typhimurium were utilized for mutagenicity assay in absence and presence of metabolic activation, i.e., with and without S9 fraction. The tester strain used were selected because they are sensitive and detect a large proportion of known bacterial mutagens and are most commonly used routinely within the pharmaceutical industry (30,31). A specific positive control was used with and without S9 fraction. 2-Nitrofluorene (2-NF) 50 µg/mL + 4-nitroquinoline N-oxide (4-NQO) 2.5 µg/mL were used as positive controls without metabolic activation, and 2-aminoanthracene (2-AA) was used as positive control with metabolic activation, respectively. Approximately 107 bacteria were exposed to six concentrations of Pcex, as well as to positive and negative controls, for 90 minutes in medium containing sufficient histidine to support approximately two cell divisions. After 90 minutes, the exposure cultures were diluted in pH indicator medium lacking histidine, and aliquoted into 48 wells of a 384-well plate. Within 2 days, cells which had undergone the reversion to His grew into colonies. Metabolism by the bacterial colonies reduced the pH of the medium, changing the colour of that well. This colour change can be detected visually. The number of wells containing revertant colonies were counted for each dose and compared to a zero dose control. Each dose was tested in six replicate.
The material was regarded mutagenic if the number of histidine revertant colonies was twice or more than the spontaneous revertant colonies.
Blood compatibility: thrombin time (TT)
In particular, 0.2 mL of each samples were added to 0.2 mL of human plasma. TT was determined by incubating the aliquot (0.2 mL) of PPP containing the sample at 37 °C for 2 minutes, after which 0.2 mL of thrombin (0.6 NIH) was added. The clotting time was revealed by an Automatic Elvi Digiclot 2 Coagulimeter (from Logos SpA, Milan, Italy).
Statistical analysis
All assays were carried out in six replicates and their results were expressed as mean ± standard deviation (SD). Multiple comparisons were performed by one-way ANOVA and individual differences tested by Fisher’s test after the demonstration of significant intergroup differences by ANOVA. Differences with P<0.05 were considered significant.
Results
Extraction yield, total phenolic content and hydrogen peroxide scavenging activity
As reported in Table 1, the concentration of dry extract obtained was 112 mg, and 1 mL of suspension contained 11.2 mg of dry material corresponding to a percentage yield of 5.6%.

Full table
Total phenolic content of Pcex, as determined by FBBB test, was 17.2±0.9 mg GAE/g of dry material and, as shown in Table 2, the extract had the capability to scavenge hydrogen peroxide in a concentration dependent manner (0.012–0.44 mg/mL) with a half maximum effective concentration (EC50) of 0.11 mg/mL corresponding at a total phenolic content of about 0.036 mg/mL. A significant strong correlation between H2O2 scavenging activity and total phenolic content of the extract was also demonstrated by the positive value of R which was equal to 0.9876.

Full table
These results demonstrated that the activities of Pcex among its constituents provided a strong contribution to the in vitro antioxidant efficiency.
Antiradical activity: EPR analysis
The antioxidant behaviour of the Pcex was evaluated by its ability to scavenge the free DPPH radical by electron donation, by EPR analysis.
The addition of the antioxidant to the DPPH radical solution determined a reduction in intensity of the signal with a scavenger percentage equal to 56%. Pcex showed a GAE antioxidant activity towards DPPH radical equal to 0.0117±0.006 (SD). The effect of Pcex on DPPH radical is reported in Figure 1 where the radical alone is shown in (Figure 1A) and the DPPH after the addition of the extract is shown in (Figure 1B).
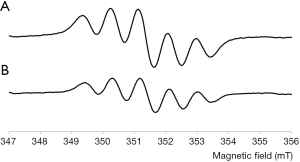
In vitro cytotoxicity
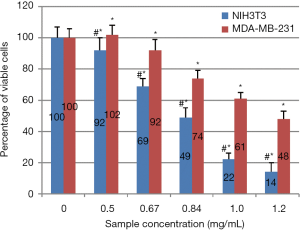
Non-confluent adhered NIH3T3 and MDA-MB-231 were incubated with different concentrations of Pcex diluted 1:5 with 60% ethanol. Cells were analysed after 24 hours of contact with the test samples and the results are reported in Figure 2. As shown, Pcex exerted an anti-proliferative effect against both NIH3T3 and MDA-MB-231 cell lines in a concentration dependent manner, with lesser efficiency on breast adenocarcinoma cells compared to fibroblasts. In fact, at all the concentration values tested, the percentage of viable NIH3T3 was statistically lower compared to MDA-MB-231. Moreover, the concentration value of 0.84 mg/mL reduced NIH3T3 viability by 50%, while the same effect with MDA-MB-231 was obtained at a Pcex concentration of 1.2 mg/mL demonstrating that cell viability was selectively influenced by the extract.
Hydrogen peroxide treatment of cells
Concentration-effect relationship for the cytotoxic action of H2O2 in NIH3T3 fibroblasts and in human breast adenocarcinoma cells MDA-MB-231 were obtained by testing concentration values of the hydrogen peroxide ranging from 0.082 to 1.6 mM. Cytotoxicity was determined as decreasing of cell viability after H2O2 treatment followed and not by contact with 0.5 mg/mL Pcex, the concentration value at which the extract showed the lowest degree of cytotoxicity for both cell lines.
Figure 3 shows that cell viability decreased by increasing hydrogen peroxide concentration with and without addition of the extract.
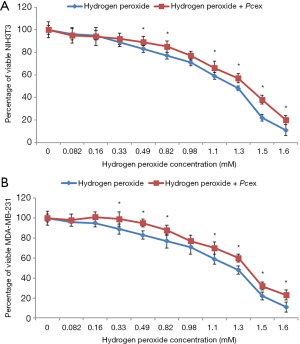
As shown in Figure 3A, Pcex was able to increase slightly NIH3T3 cell viability after treatment with H2O2 and only for concentrations ranging from 0.82 and 1.6 mM (Figure 3A).
The addition of Pcex increased the percentage of viable MDA after treatment with hydrogen peroxide in a range of concentrations between 0.33 and 1.6 mM (Figure 3B). Human adenocarcinoma breast cells demonstrated to be more resistant to H2O2 treatment in comparison to NIH3T3 fibroblasts and the extract improved cell viability neutralizing the toxic effect of H2O2. In this case Pcex interfered with the H2O2 toxicity also at the highest concentration, demonstrating to be able to protect cells and to reduce cell death.
Mutagenicity assay: Ames test
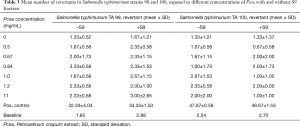
In Salmonella typhimurium mutagenicity assay, six different concentrations of Pcex were tested by Ames test on TA98, and TA100 strains with and without S9 metabolic activation. The results for the mutagenic effect of Pcex reported in Table 3 demonstrated that all the concentrations tested were not toxic towards both TA98 and TA100 with and without S9 fraction. In fact, also at the highest concentration (11 mg/mL), the number of revertants was lower and statistically different in comparison to positive control (P<0.01). The background level as well as positive control values were in all cases within the normal limit found in our laboratory and in accordance with literature data.
Full table
Blood compatibility: TT
TT looks at the transformation of fibrinogen to fibrin by thrombin. By looking at the TT values reported in Table 4, we note that all the five Roe concentrations tested significantly increased clotting time in comparison to the control (plasma diluted 1:1 with EtOH 60%) demonstrating to have an anti-coagulating effect also at the lowest concentration value.

Full table
Conclusions
The data obtained demonstrated that hydro-alcoholic extraction of P. crispum can be a good method for obtaining active compounds with high bioactivity. The results of this study have demonstrated, in fact, that the Pcex analysed possessed good antioxidant and radical scavenging activities when tested in cellular and non-cellular assays, absence of genotoxic and ability to prolonge TT. Moreover, NIH3T3 mouse fibroblasts and human breast adenocarcinoma cells MDA-MB-231 viability was selectively influenced by the extract. All these bioactivities are tightly correlated to the Pcex phenolic content in a dose dependent manner.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/lcm-20-47
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-20-47). SL serves as an unpaid editorial board member of Longhua Chinese Medicine from Jul 2020 to Jun 2022. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived. Ethical approval was not required for this study design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tlili H, Hanen N, Ben Arfa A, et al. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS One 2019;14:e0213049 [Crossref] [PubMed]
- Essawi T, Srour M. Screening of some Palestinian medicinal plants for antibacterial activity. J Ethnopharmacol 2000;70:343-9. [Crossref] [PubMed]
- Parveen M, Malla AM, Alam M, et al. Two new phenolic compounds from Ficus rumphii and their antiproliferative activity. Nat Prod Res 2014;28:646-52. [Crossref] [PubMed]
- El-Seedi HR, Burman R, Mansour A, et al. The traditional medical uses and cytotoxic activities of sixtyone Egyptian plants: discovery of an active cardiac glycoside from Urginea maritima. J Ethnopharmacol 2013;145:746-57. [Crossref] [PubMed]
- Ouelbani R, Bensari S, Mouas TN, et al. Ethnobotanical investigations on plants used in folk medicine in the regions of Constantine and Mila (North-East of Algeria). J Ethnopharmacol 2016;194:196-218. [Crossref] [PubMed]
- Kumar V, Marković T, Emerald M, et al. Herbs: Composition and dietary importance. In: Caballero B, Finglas PM, Toldrá F. editors. Encyclopedia of food and health. Oxford: Academic Press, 2016:332-7.
- Agyare C, Appiah T, Boakye YD, et al. Chapter 25: Petroselinum crispum: a review. In: Kuete V. Medicinal spices and vegetables from Africa. Cambridge: Academic Press 2017:527-47.
- Mahmood S, Hussain S, Malik F. Critique of medicinal conspicuousness of Parsley(Petroselinum crispum): a culinary herb of Mediterranean region. Pak J Pharm Sci 2014;27:193-202. [PubMed]
- Mert A, Timur M. Essential oil and fatty acid composition and antioxidant capacity and total phenolic content of parsley seeds (Petroselinum crispum) grown in Hatay region. Indian J Pharm Educ Res 2017;51:S437-40. [Crossref]
- Angioni A, Barra A, Cereti E, et al. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J Agric Food Chem 2004;52:3530-5. [Crossref] [PubMed]
- Bendimerad N, Taleb Bendiab SA, Benabadji AB, et al. Composition and antibacterial activity of Pseudocytisus integrifolius (Salisb.) essential oil from Algeria. J Agric Food Chem 2005;53:2947-52. [Crossref] [PubMed]
- Kim HJ, Chen F, Wu C, et al. Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J Agric Food Chem 2004;52:2849-54. [Crossref] [PubMed]
- Miguel G, Simões M, Figueiredo AC, et al. Composition and antioxidant activities of the essential oils of Thymus caespititius, Thymus camphoratus and Thymus mastichina. Food Chem 2004;86:183-8. [Crossref]
- Al-Haadi A, Al Rahbi S, Akhtar M, et al. Phytochemical screening, antibacterial and cytotoxic activities of petroselinum crispum leaves grown in Oman. Iran J Pharm Res 2013;9:61-5.
- O'Mahony R, Al-Khtheeri H, Weerasekera D, et al. Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J Gastroenterol 2005;11:7499-507. [Crossref] [PubMed]
- Tang EL, Rajarajeswaran J, Fung S, et al. Petroselinum crispum has antioxidant properties, protects against DNA damage and inhibits proliferation and migration of cancer cells J Sci Food Agric 2015;95:2763-71. [Crossref] [PubMed]
- El-Sayed M, Metwally N, Ibrahim I, et al. Antioxidant activity, total phenolic and flavonoid contents of Petroselinum crispum mill. JALSI 2018;19:1-7. [Crossref]
- Fernández Ochoa Á, Borrás I, Pérez-Sánchez A, et al. Phenolic compounds in rosemary as potential source of bioactive compounds against colorectal cancer: In situ absorption and metabolism study. J Func Food 2017;33:202-10. [Crossref]
- Ribeiro A, Caleja C, Barros L, et al. Rosemary extracts in functional foods: Extraction, chemical characterization and incorporation of free and microencapsulated forms in cottage cheese. Food Funct 2016;7:2185-96. [Crossref] [PubMed]
- Mulinacci N, Innocenti M, Bellumori M, et al. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: an HPLC/DAD/MS study. Talanta 2011;85:167-76. [Crossref] [PubMed]
- Bicchi C, Binello A, Rubiolo P. Determination of phenolic diterpene antioxidants in rosemary (Rosmarinus officinalis L.) with different methods of extraction and analysis. Phytochem Anal 2000;11:236-42. [Crossref]
- Lester G, Lewers K, Medina M, et al. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin-Ciocalteu: assay interference by ascorbic acid. J Food Compost Anal 2012;27:102-7. [Crossref]
- Hinojosa-Nogueira D, Muros J, Rufián-Henares JA, et al. New Method to estimate total polyphenol excretion: comparison of Fast Blue BB versus folin-ciocalteu performance in urine. J Agric Food Chem 2017;65:4216-22. [Crossref] [PubMed]
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989;10:1003-8. [Crossref] [PubMed]
- Tamasi G, Baratto MC, Bonechi C, et al. Chemical characterization and antioxidant properties of products and by-products from Olea europaea L. Food Sci Nutr 2019;7:2907-20. [Crossref] [PubMed]
- ISO 10995-5:2009. Biological evaluation of medical devices--Part 5: tests for cytotoxicity: in vitro methods.
- Lamponi S, Leone G, Consumi M, et al. In vitro biocompatibility of new PVA-based hydrogels as vitreous body substitutes. J Biomater Sci Polym Ed 2012;23:555-75. [Crossref] [PubMed]
- Lamponi S, Leone G, Consumi M, et al. Porous multi-layered composite hydrogel as cell substrate for in vitro culture of chondrocytes. Int J Polym Mater 2020; [Crossref]
- Lamponi S, Aloisi AM, Bonechi C, et al. Evaluation of in vitro cell and blood compatibility and in vivo analgesic activity of plant-derived dietary supplements. J Integr Med 2019;17:213-20. [Crossref] [PubMed]
- Purves D, Harvey C, Tweats D, et al. Genotoxicity testing: current practices and strategies used by the pharmaceutical industry. Mutagenesis 1995;10:297-312. [Crossref] [PubMed]
- Makhafola TJ, Elgorashi EE, McGaw LJ, et al. The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant soecies with high antioxidant activity. BMC Complement Altern Med 2016;16:490. [Crossref] [PubMed]
Cite this article as: Lamponi S, Baratto MC. Bioactivity of hydro-alcoholic extract of Petroselinum crispum. Longhua Chin Med 2020;3:16.

