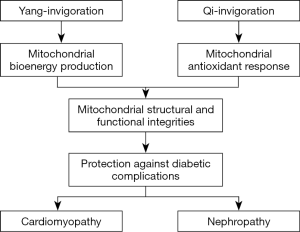
Yang/Qi-invigorating herbs for the prevention and/or treatment of diabetic complications
The Yin-Yang theory in traditional Chinese medicine (TCM)
TCM views the human body as an organic entity made up of various organs that function in a mutually interdependent manner (1). The Yin-Yang theory, which originated from ancient Chinese philosophy, is central to the conceptual framework of TCM. According to the Yin-Yang theory, the functioning of the human body is governed by the interplay of two complementary, but opposing, forces, namely, Yin and Yang. The dynamic equilibrium between Yin and Yang determines the physiological status of the human body (2). Any disturbance in the balance of Yin and Yang can lead to various pathological conditions. For example, an excess of Yin can lead to an over-consumption of Yang and, conversely, a relative excess of Yin or Yang is referred to as Yang deficiency or Yin deficiency, respectively. Therefore, within this conceptual framework, TCM classifies bodily structures, explains clinical symptoms, and guides the treatment of diseases on the basis of the Yin-Yang theory (3).
According to TCM theory, the interaction between Yin (postnatal essence or Yin Qi) and Yang (prenatal essence or Yang Qi) generates Zheng Qi (i.e., “the Qi of the body”) which maintains life activities and defends against pathogens, as well as allowing adaptive changes in the body in response to internal and/or external factors. Zheng Qi can flow through meridians and thereby energize physiological activities. TCM theory also states that aging and aging-associated disorders can result in a gradual depletion of Zheng Qi and the complete deprivation of Zheng Qi (which is mainly due to the exhaustion of prenatal essence) will ultimately result in death (4). As the prenatal essence is inherited from parents and hence cannot be replenished after birth, a sufficient supply of postnatal essence is needed to minimize the utilization of prenatal essence for the generation of Zheng Qi. According to TCM theory, Blood, a thick red liquid circulating in circulatory vessels, is viewed as one of the basic substances essential for supporting vital activities in humans. Blood circulation in the body is dependent on the driving action of Qi and it exerts a strong nourishing effect on various organs. The inter-relationship between Qi and Blood exemplifies the importance of a harmony between Yin (Blood) and Yang (Qi) in determining optimal physiological function.
Restoration of Yin-Yang balance in sub-healthy conditions
While exhaustive sexual activity and the Yin-Yang imbalance of body functioning can cause an over-consumption of prenatal essence, the maintenance of an optimal Yin-Yang balance for body functions (in particular that of the “Spleen” for digestion and absorption) and a healthy lifestyle (diet and physical exercise) is crucial in acquiring sufficient postnatal essence, and thereby in preserving the prenatal essence in humans. In this regard, the use of Chinese tonifying herbs aims to re-establish the Yin-Yang balance in the body and thereby prevent the over-consumption of prenatal essence. In addition, any internal or external causes of diseases (i.e., pathogenic factors) can be classified as Yin or Yang on the basis of their pathological characteristics. Various modalities of interventions (therapeutic and tonifying) in TCM, including the use of herbal formulations and acupuncture, can also exert Yin/Yang-modulatory actions, thereby producing beneficial effects in Yin/Yang-deficiency disorders (3). While Chinese herbs are broadly divided into therapeutic and tonifying groups in terms of their pharmacological actions, tonifying herbs can be further divided into four functional categories: Yang-invigorating, Yin-nourishing, Qi-invigorating and Blood-enriching, with respect to their mode of action in enhancing physiological functions under sub-optimal body conditions (5). With regard to the Yin-Yang theory, Qi-invigorating and Blood-enriching actions are classified under the Yang and Yin categories, respectively.
Differing modes of action of Yang and Qi-invigorating herbs
Various body functions are dependent on cellular activities, which are manifestations of Yang and Qi. In this regard, mitochondria (the “powerhouse” of the cell) generates ATP that energizes a wide range of cellular activities. To keep up with their ATP generation capacity (ATP-GC), a glutathione-dependent antioxidant system in mitochondria is in place to protect respiratory complexes against reactive oxygen species (ROS) arising from mitochondrial electron transport (6,7). With this in mind, the stimulation of mitochondrial ATP generation by the Yang/Qi-invigorating action of Chinese tonifying herbs may involve an increase in electron transport and/or the induction of a glutathione antioxidant response which can safeguard mitochondrial ATP-GC.
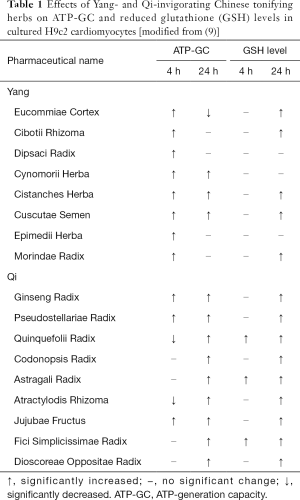
The pharmacological basis of Yang-invigoration has been demonstrated by our laboratory, wherein Yang-invigorating herbal extracts consistently increase mitochondrial ATP-GC both ex vivo in mouse hearts and in vitro in H9c2 cells (5,8). A recent study in our laboratory has further compared the capacity of Yang-invigorating and Qi-invigorating herbal extracts (Table 1) to stimulate mitochondrial ATP-GC and increase cellular reduced glutathione (GSH) levels in cultured H9c2 cardiomyocytes (10). Qi-invigorating herbal extracts were found to increase mitochondrial ATP-GC in H9c2 cells after a long (24 h) versus a short (4 h) incubation, as was the case for Yang-invigorating herbal extracts (Table 1). The experimental result points to the possibility that Yang-invigorating and Qi-invigorating herbs stimulate mitochondrial ATP-GC via different mechanisms of action. In this connection, an earlier study in our laboratory has demonstrated that β-sitosterol, an active component of Cistanches Herba (a Yang-invigorating tonifying herb), can fluidize mitochondrial inner membrane and stimulate mitochondrial ATP-GC in cultured C2C12 myocytes (9). This postulation is strengthened by the observation that the Yang-invigorating herbal extract-induced stimulation of ATP-GC is completely abrogated by cholesterol (a biomembrane stabilizer). Presumably, the fluidization of mitochondrial inner membrane by active components of Yang-invigorating herbal extracts can activate enzymes involved in electron transport (9), resulting in the increase in mitochondrial ATP-GC under the described experimental conditions.
Full table
On the other hand, Qi-invigorating herbal extracts were found not able to stimulate mitochondrial ATP-GC following a 4 h incubation, whereas all of them stimulated ATP-GC in H9c2 cells following incubation for 24 h (Table 1). The stimulation of mitochondrial ATP-GC was associated with an increase in cellular GSH levels. Regression analysis showed a positive significant correlation between the degrees of mitochondrial ATP-GC stimulation and the extents of cellular GSH increase in Qi-invigorating herbal extract-pre-incubated H9c2 cells. The observation that co-incubation of H9c2 cells with inhibitors of glutathione synthesis and glutathione regeneration abolished the stimulatory effect of Qi-invigorating herbal extracts on mitochondrial ATP-GC further confirms that the increase in mitochondrial ATP-GC is secondary to an enhancement of cellular GSH status. This is consistent with earlier findings that glutathione antioxidant status plays a crucial role in maintaining mitochondrial structural and functional integrity (11,12). Recently, studies on antioxidant phytochemicals have revealed that various phytochemicals (such as curcumin, catechin, resveratrol and schisandrin B) can serve as direct free radical scavengers and/or induce a nuclear factor (erythroid-derived 2)-like 2 (Nrf2)-driven expression of antioxidant proteins/enzymes (13,14). With respect to the mechanism underlying the enhancement of cellular GSH levels by Qi-invigorating herbs, Qi-invigorating herbs may enable the maintenance of cellular GSH levels by free radical scavenging activities and/or increasing the expression of glutathione-related proteins/enzymes.
Protection against diabetic cardiomyopathy by Yang/Qi-invigoration
Cardiomyopathy is a major cause of morbidity and mortality in patients with diabetes. Recent studies have shown that mitochondrial dynamics and mitophagy (a cellular event involving the removal of dysfunctional mitochondria are sensitive to nutrient availability and hence their dysregulation is associated with diabetes. As such, the induction of mitochondrial fusion [which is mediated by mitofusin 1 (Mfn1), mitofusin 2 (Mfn2) and optic atrophy 1 (Opa1)] via the up-regulation of Mfn2 was found to alleviate high-fat diet (HFD)-induced insulin resistance and adiposity in diabetic rodents (15). The induction of mitochondrial fusion by incubation with insulin can also enhance energy GC and increase the rate of oxygen consumption in cultured cardiomyocytes (16). In contrast, increased mitochondrial fission [which is mediated by dynamin-related protein 1 (Drp1), mitochondrial fission factor (Mff) and fission 1 (Fis1)] was observed in coronary endothelial cells isolated from diabetic mouse hearts (17). Over-nutrition, as occurs with high glucose and lipid diets, can cause an excessive production of ROS during mitochondrial oxidative phosphorylation in cardiomyocytes, with resultant oxidative damage to mitochondria. An impairment in mitophagy was found to be associated with cardiomyopathy, probably due to an increased number of dysfunctional mitochondria in the heart (18). Mitophagy involves the fusion of fragmented mitochondria with autophagosomes, in which LC-3/p62 are crucial regulators of the process (18). The restoration of mitophagy by pharmacological intervention was found to improve the cardiomyopathy in diabetic mice (19). The aforementioned findings suggest that the control of mitochondrial quality (i.e., functional integrity) is critical in the pathogenesis of cardiomyopathy.
Mitohormesis, which refers to a phenomenon wherein mild oxidative stress in mitochondria can protect the cell/tissue against subsequent perturbations, is proposed to promote longevity and protect against aging-associated diseases (20). For instance, caloric restriction, physical exercise or treatment with phytochemicals have been shown to induce a mitohormetic response via modulating mitochondrion-related and bioenergetics-sensitive signaling regulators, such as 5' adenosine monophosphate-activated protein kinase (AMPK), sirtuin(s) as well as the target of rapamycin (mTOR) (21). AMPK and sirtuin(s), which act antagonistically with mTOR, can activate a number of transcription factors regulating mitochondrial function, such as peroxisome proliferator-activated receptor gamma coactivator (PGC1) α and β, peroxisome proliferator-activated receptors (PPAR) α and γ and Forkhead box O3 (Foxo3) (21). While PGC 1α and β can regulate the expression of Mfn2 (22), which in turn can improve the insulin signaling cascade (15), the induction of PPARα can facilitate fatty acid β-oxidation (23). Emerging evidence has also shown a possible correlation between mitohormetic signaling and the maintenance of mitochondrial functional integrity. As such, recent studies have shown that the activation of PPARγ and/or Foxo3 can induce BNIP3/LC-3/p62-mediated mitophagy in heart tissues (24). In addition, a small degree of mitochondrial uncoupling (i.e., the uncoupling of oxidative phosphorylation) can reduce ROS production in mitochondria and thereby attenuate the oxidative damage. In support of this, a recent study has shown that Nrf2, the master regulator of antioxidant response plays a role in mitohormesis (induced by a small degree of mitochondrial uncoupling) in skeletal muscle of transgenic mice deficient in oxidative phosphorylation capacity (25).
Recent studies in our laboratory have shown that Cistanches Herba and Cynomorii Herba (two Yang-invigorating herbs) can fluidize the mitochondrial inner membrane, causing a small increase in ROS production. This increase in ROS generation can induce a small degree of uncoupling effect which serves as a retrograde signal for the induction of an antioxidant response in the cultured H9c2 cardiomyocytes (26,27). In this connection, the induction of the glutathione-dependent antioxidant response elicited by Cistanches Herba and Cynomorii Herba was found to be associated with cyto- and cardio-protection against oxidative stress in cultured menadione-intoxicated H9c2 cardiomyocytes as well as in ischemic/reperfused rat hearts (26,27). In addition, Cistanches Herba and Cynomorii Herba can induce a mitohormetic response, via activation of the AMPK/PGC-1 pathway. As such, Cistanches Herba can cause a redox-sensitive induction of mitochondrial uncoupling and activation of AMPK/PGC-1 in C2C12 Myotubes and reduce the body weight of HFD-fed obese mice (9). Ursolic acid, the active ingredient of Cynomorii Herba, can induce mitochondrial biogenesis through the activation of AMPK/PGC-1 in C2C12 myotubes and increase the exercise endurance capacity in mice (28). Ginsenoside Rg1, which is one of the active ingredients in Ginseng Radix (a Qi-invigorating herb), was found to reduce oxidative stress and the degree of cardiomyopathy in streptozotocin-injected diabetic rats via the induction of an antioxidant response (29). A Yin-nourishing and Qi-invigorating herbal formula, namely Shengmai San, was found to reduce the extent of myocardial fibrosis in streptozotocin-diabetic rats (30), presumably via the activation of mitohormetic AMPK and sirtuin 1 signaling pathways (31).
Protection against diabetic nephropathy by Yang/Qi-invigoration
The kidneys, which are highly sensitive to hemodynamic and metabolic alterations, are susceptible to microvascular damage in patients with diabetes. Diabetic nephropathy, one of the major microvascular complications of diabetes, is characterized by albuminuria, a reduction in glomerular filtration rate and ultimately a decline in renal functions (i.e., end-stage renal disease) (32). Recent studies have shown that oxidative stress and inflammation arising from hyperglycemia play critical roles in the pathogenesis of diabetic nephropathy (33). As described in the previous section, oxidative stress originates mainly from the excessive production of ROS arising from mitochondrial oxidative phosphorylation. The oxidant-induced damage in the glomerulus can induce leukocyte infiltration, with a resultant inflammatory response (33). Transforming growth factor-β1, one of the pro-inflammatory mediators secreted by renal cells upon interaction with leukocytes, has been identified as the key factor involved in the pathogenesis of diabetic nephropathy (34). TGF-β1 can promote the de-differentiation of epithelial-mesenchymal cells (which normally possess a highly polarized structure for efficient reabsorption of solutes and proteins from the renal tubule into peritubular capillaries), which in turn reduces cell polarity and hence the capability of reabsorption of solutes in the nephron (35). The tubular dysfunction ultimately leads to albuminuria, polyuria and glycosuria. TGF-β1 can also facilitate the accumulation of components of the extracellular matrix (such as collagen, fibronectin and laminin) in the glomerulus, with resultant glomerulosclerosis and tubulointerstitial fibrosis (36).
Recent studies exploring the possible cause-and-effect relationship between oxidative stress and diabetic nephropathy have demonstrated that the induction of a Nrf2-driven antioxidant response can ameliorate pathological manifestations of diabetic nephropathy in experimental animals whereas, conversely, the deletion of the Nrf2 gene can augment the severity of diabetic nephropathy (37). In an effort to attenuate the progression of oxidant-mediated diabetic nephropathy, interventions with antioxidant and anti-inflammatory activity may offer a reasonable approach. Given that both Yang- and Qi-invigorating herbs can induce a glutathione-dependent antioxidant response, they should prove effective in the prevention and/or treatment of diabetic nephropathy. A previous finding in our laboratory has shown that a Yang-invigorating herbal health product (namely, VI-28) can protect against gentamicin-induced nephrotoxicity in rats associated with increases in the level/activities of GSH, superoxide dismutase, Se-glutathione peroxidase and glutathione S-transferases, all of which are regulated by Nrf2 (38). With regard to compounds isolated from Qi-invigorating herbs, ginsenoside Rg1 (alone or in combination with astragaloside IV) can suppress oxidative stress and reduce the severity of nephropathy in streptozotocin-diabetic rats via the inhibition of TGF-β1/Smads signaling cascades (39). Similarly, schisandrin B, an active ingredient isolated from Schisandra Fructus (also regarded as an herb that can invigorate the Qi of the five viscerae), can alleviate hyperglycemia-induced renal injury in streptozotocin-diabetic mice, by suppressing inflammation and oxidative stress (40).
Conclusions
In conclusion, the health-promoting actions of, Yang-invigorating herbs are mediated, at least in part, by increasing mitochondrial ATP-GC, likely by fluidizing the mitochondrial inner membrane, whereas Qi-invigorating herbs increase mitochondrial ATP-GC via the induction of a glutathione-dependent antioxidant response, which can indirectly preserve the functional integrity of mitochondria (Figure 1). These observations strongly support the view that mitochondria are the primary target of Yang- and Qi-invigoration. The possible beneficial effects of Yang- and Qi-invigorating herbs in the prevention and/or/treatment of diabetic cardiomyopathy and nephropathy warrants further investigation. Future studies should be focused on identifying active compounds from Yang- and Qi-invigorating herbs in relation to their action in enhancing mitochondrial ATP-GC and glutathione antioxidant status. The molecular mechanisms underlying Yang- and Qi-invigoration can then be elucidated. Consequently, biomarkers can be established for implementing quality control for Yang/Qi-invigorating herbs and related herbal health products.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm.2019.03.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- O’Brien KA, Xue CC. The theoretical framework of Chinese medicine. In: Leung PC, Xue CC, Chen YC. Editors. A comprehensive guide to Chinese medicine. Singapore: World scientific publishing Co. Pte. Ltd, 2003:47-84.
- Liu Z, Liu L. Essentials of Chinese medicine. London: Springer, 2009.
- Zhang D, Wu X. Qi, Blood, Body fluid, Essence of life and spirit. In: Liu Y. Editor. The basic knowledge of traditional Chinese medicine. Hong Kong: Hai Feng Publishing Co., 1991:49-53.
- Leong PK, Wong HS, Chen J, et al. Yang/Qi invigoration: an herbal therapy for chronic fatigue syndrome with Yang deficiency? Evid Based Complement Alternat Med 2015;2015:945901 [Crossref] [PubMed]
- Ko KM, Leon TY, Mak DH, et al. A characteristic pharmacological action of 'Yang-invigorating' Chinese tonifying herbs: enhancement of myocardial ATP-generation capacity. Phytomedicine 2006;13:636-42. [Crossref] [PubMed]
- Kolossov VL, Beaudoin JN, Ponnuraj N, et al. Thiol-based antioxidants elicit mitochondrial oxidation via respiratory complex III. Am J Physiol Cell Physiol 2015;309:C81-91. [Crossref] [PubMed]
- Dröse S, Brandt U, Wittig I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim Biophys Acta 2014;1844:1344-54. [Crossref] [PubMed]
- Ko KM, Leung HY. Enhancement of ATP generation capacity, antioxidant activity and immunomodulatory activities by Chinese Yang and Yin tonifying herbs. Chin Med 2007;2:3. [Crossref] [PubMed]
- Wong HS, Leong PK, Chen J, et al. β-Sitosterol increases mitochondrial electron transport by fluidizing mitochondrial membranes and enhances mitochondrial responsiveness to increasing energy demand by the induction of uncoupling in C2C12 myotubes. J Funct Foods 2016;23:253-60. [Crossref]
- Leong PK, Leung HY, Chan WM, et al. Differences in the Mechanisms by Which Yang-Invigorating and Qi-Invigorating Chinese Tonifying Herbs Stimulate Mitochondrial ATP Generation Capacity. Chin Med 2018;9:63-74. [Crossref]
- Yu H, Liu J, Li J, et al. Protection of mitochondrial integrity from oxidative stress by selenium-containing glutathione transferase. Appl Biochem Biotechnol 2005;127:133-42. [Crossref] [PubMed]
- Marí M, Morales A, Colell A, et al. Mitochondrial Glutathione, a Key Survival Antioxidant. Antioxid Redox Signal 2009;11:2685-700. [Crossref] [PubMed]
- Stefanson AL, Bakovic M. Dietary Regulation of Keap1/Nrf2/ARE Pathway: Focus on Plant-Derived Compounds and Trace Minerals. Nutrients 2014;6:3777-801. [Crossref] [PubMed]
- Leong PK, Ko KM. Induction of the Glutathione Antioxidant Response/Glutathione Redox Cycling by Nutraceuticals: Mechanism of Protection against Oxidant-induced Cell Death. Curr Trends Nutraceuticals 2016;1:112.
- Gan KX, Wang C, Chen JH, et al. Mitofusin-2 ameliorates high-fat diet-induced insulin resistance in liver of rats. World J Gastroenterol 2013;19:1572-81. [Crossref] [PubMed]
- Parra V, Verdejo HE, Iglewski M, et al. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt-mTOR-NFκB-Opa-1 signaling pathway. Diabetes 2014;63:75-88. [Crossref] [PubMed]
- Makino A, Suarez J, Gawlowski T, et al. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol 2011;300:R1296-302. [Crossref] [PubMed]
- Bai T, Wang F, Zheng Y, et al. Myocardial redox status, mitophagy and cardioprotection: a potential way to amend diabetic heart? Clin Sci (Lond) 2016;130:1511-21. [Crossref] [PubMed]
- Wang S, Zhao Z, Feng X, et al. Melatonin activates Parkin translocation and rescues the impaired mitophagy activity of diabetic cardiomyopathy through Mst1 inhibition. J Cell Mol Med 2018;22:5132-44. [Crossref] [PubMed]
- Ristow M, Schmeisser K. Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose Response 2014;12:288-341. [Crossref] [PubMed]
- Sebastián D, Palacín M, Zorzano A. Mitochondrial Dynamics: Coupling Mitochondrial Fitness with Healthy Aging. Trends Mol Med 2017;23:201-15. [Crossref] [PubMed]
- Zorzano A. Regulation of mitofusin-2 expression in skeletal muscle. Appl Physiol Nutr Metab 2009;34:433-9. [Crossref] [PubMed]
- Hsu WH, Lee BH, Pan TM. Leptin-induced mitochondrial fusion mediates hepatic lipid accumulation. Int J Obes (Lond) 2015;39:1750-6. [Crossref] [PubMed]
- Chaanine AH, Kohlbrenner E, Gamb SI, et al. FOXO3a regulates BNIP3 and modulates mitochondrial calcium, dynamics, and function in cardiac stress. Am J Physiol Heart Circ Physiol 2016;311:H1540-59. [Crossref] [PubMed]
- Coleman V, Sa-Nguanmoo P, Koenig J, et al. Partial involvement of Nrf2 in skeletal muscle mitohormesis as an adaptive response to mitochondrial uncoupling. Sci Rep 2018;8:2446. [Crossref] [PubMed]
- Chen J, Wong HS, Ko KM. Ursolic Acid-enriched Herba Cynomorii extract induces mitochondrial uncoupling and glutathione redox cycling through mitochondrial reactive oxygen species generation: protection against menadione cytotoxicity in h9c2 cells. Molecules 2014;19:1576-91. [Crossref] [PubMed]
- Wong HS, Chen N, Leong PK, et al. β-Sitosterol enhances cellular glutathione redox cycling by reactive oxygen species generated from mitochondrial respiration: protection against oxidant injury in H9c2 cells and rat hearts. Phytother Res 2014;28:999-1006. [Crossref] [PubMed]
- Chen J, Wong HS, Leong PK, et al. Ursolic acid induces mitochondrial biogenesis through the activation of AMPK and PGC-1 in C2C12 myotubes: a possible mechanism underlying its beneficial effect on exercise endurance. Food Funct 2017;8:2425-36. [Crossref] [PubMed]
- Yu HT, Zhen J, Pang B, et al. Ginsenoside Rg1 ameliorates oxidative stress and myocardial apoptosis in streptozotocin-induced diabetic rats. J Zhejiang Univ Sci B 2015;16:344-54. [Crossref] [PubMed]
- Ni Q, Wang J, Li EQ, et al. Study on the protective effect of shengmai san (see text) on the myocardium in the type 2 diabetic cardiomyopathy model rat. J Tradit Chin Med 2011;31:209-19. [Crossref] [PubMed]
- Tian J, Tang W, Xu M, et al. Shengmai San Alleviates Diabetic Cardiomyopathy Through Improvement of Mitochondrial Lipid Metabolic Disorder. Cell Physiol Biochem 2018;50:1726-39. [Crossref] [PubMed]
- Toth-Manikowski S, Atta MG. Diabetic Kidney Disease: Pathophysiology and Therapeutic Targets. J Diabetes Res 2015;2015:697010 [Crossref] [PubMed]
- Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 2014;20:1126-67. [Crossref] [PubMed]
- Chang AS, Hathaway CK, Smithies O, et al. Transforming growth factor-β1 and diabetic nephropathy. Am J Physiol Renal Physiol 2016;310:F689-96. [Crossref] [PubMed]
- Hills CE, Squires PE. The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev 2011;22:131-9. [PubMed]
- Ignotz RA, Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 1986;261:4337-45. [PubMed]
- Ruiz S, Pergola PE, Zager RA, et al. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 2013;83:1029-41. [Crossref] [PubMed]
- Poon MK, Chiu PY, Leung HY, et al. A 'Yang-invigorating' Chinese herbal formula protects against gentamicin-induced nephrotoxicity in rats. Phytother Res 2008;22:131-3. [Crossref] [PubMed]
- Du N, Xu Z, Gao M, et al. Combination of Ginsenoside Rg1 and Astragaloside IV reduces oxidative stress and inhibits TGF-β1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy. Drug Des Devel Ther 2018;12:3517-24. [Crossref] [PubMed]
- Mou Z, Feng Z, Xu Z, et al. Schisandrin B alleviates diabetic nephropathy through suppressing excessive inflammation and oxidative stress. Biochem Biophys Res Commun 2019;508:243-9. [Crossref] [PubMed]
Cite this article as: Leong PK, Ko KM. Yang/Qi-invigorating herbs for the prevention and/or treatment of diabetic complications. Longhua Chin Med 2019;2:4.