
A Narrative review of scientific validation of gold- and silver-based Indian medicines and their future scope
Introduction
Metal-based medicines are used in the Indian medical systems, namely Ayurveda and Siddha. Gold (1), silver (2), copper (3), iron (4), and mercury (5) are some of the metals commonly used, where gold- and silver-based medicines are the most popular. The metal ashes are prepared through a special process that involves incineration of the metals that result in the formation of nano-and micro-scale particles. The metal ashes are dosed orally in the range of 1–5 mg/day for a few days along with certain adjuvants (plant or dairy products). Gold and silver ashes are used to treat neuropsychiatric disorders and tumor-related conditions. Most of these drugs were formulated around the 8th century (6) and, presently, researchers have shown great interest in studying them, using contemporary scientific tools such as electron microscopy and X-ray diffraction instruments, etc., as well as animal and cell models. Not only did they study the properties of these ashes (7-24) efforts were also made to provide scientific interpretation behind the unique fabrication processes (8,9,16,17,20-23). Parallelly, the pharmacokinetic routes of these drugs were studied in a quantitative manner, using in vitro and in vivo models (7,10,14,25-39). As a result of these studies, the knowledge about the fabrication process and their therapeutic efficiencies has advanced. In this article, we discuss the details of the fabrication processes that are in use since the beginning and the attempts to repurpose them as nanomedicine, using the scientific data reported on the gold and silver ashes, published from 1997–2020. The following article is presented in accordance with the Narrative Review checking checklist (available at http://dx.doi.org/10.21037/lcm-20-34).
Decoding the traditional methods used in the fabrication of metal ash
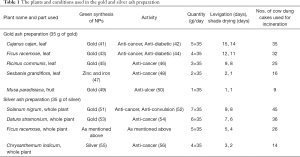
The metal-medicine is prepared by an elaborate process that involves purification, levigation and incineration. It is a top-down process; the metal foils are broken down into micro-nano scale particles. Large amounts of freshly prepared plant extracts are used during the process, and more than one method is used to prepare each metal ash (40) (Table 1). The metal ash preparation can be broadly classified as mercury containing and formulations without mercury. A few of the methods used to prepare gold and silver ashes, respectively, and their scientific interpretation are described here.
Full table
Purification process
Purification is the first step in the fabrication of metal ashes. The metal is made into a thin foil (40–60 µm thick) and is heated to incandescence. It is then quenched in vegetable oils (sesame oil, castor oil, etc.) and other liquids (cow’s urine, plant extracts, etc.). During the purification process, the metal undergoes both mechanical and chemical changes. The chemical changes mostly depend on the chemical composition of the quenchant. The metal foil is softened and sometime broken down into finer particles during the quenching (8,22,57,58). In the case of cow’s urine which is rich in urea (59), quenching releases ammonia that forms ammonium hydroxide and further react with the metals to form the respective oxides (57). The metallic shine was reported to have changed to a dullish black, post the purification process of silver, possibly due to the formation of silver oxide (22,58). The cow’s urine treatment has been shown to remove silica from the materials like Hematite and Magnetite which are recovered from the soil, and later used for preparing ashes (57). Similar reactions are believed to occur when the metal foils are purified in oils and plant extracts (22). Sesame oil (Sesamum indicum Linn.), is most commonly used during the metal purification process, probably because of its easy availability in India (58). Lime juice and other plant extracts are also used as quenchants (22), however, to what extent the compounds present in the quenchant contributes to the purification process is not known. In some cases, a slight weight change was observed at the end of purification (58). The purification process may eliminate certain impurities, or produce new compounds, or simply alter the microstructure and increase the brittleness. It is presumed that all of the above mechanical and chemical changes may be advantageous during the downstream processes.
Levigation and incineration process
The levigation is the second and the longest of the three processes. The purified metal is wet-grinded in a granite mortar and pestle along with plant extracts. The amount of plant extract is almost 150–200 times larger than the amount of metal used during levigation. For example, to grind 35 g of gold, almost 5 kg of plant extract was used (Table 1). In mercury containing formulations, mercury is included and the amalgam is wet-grinded. The process involves non-stop grinding over many days. The grinding during the levigation process determines the particle-particle and the particle-grinding media interactions, and it is the most common method used for reducing the particle size (60). In general, the particles may undergo amorphization, lattice deformation, agglomeration and increment in the surface area. The levigated material is collected and made into small cakes and shade dried, prior to incineration.
The dried levigated metal-cake is finally incinerated; it is placed inside a clay crucible and covered by mounting another crucible on the top. The junction is sealed, using a cotton cloth smeared with wet clay, limiting the supply of air inside the crucible. The cow dung cakes are used as heating fuel where, a maximum of 800–900 °C is reached (8,22,58,61). However, the temperature inside the crucible is expected to be much higher. In the recent years, electrical furnace is substituted for cow dung-based incineration (58). It is a general rule that the levigation-incineration cycle is repeated multiple times, in some cases, even 1,000 times until the right texture and color are achieved (16). The objective of the repeated cycles of levigation and incineration processes is, not only to achieve a small particle size, but also to complete certain chemical processes, in this case, the oxidation of the metals (16). In most cases, the end product is the oxide of the corresponding metal and, in cases where sulphur is included, the sulphide of the metals is also present in the metal ash (8,16,19,20,25).
In gold ash fabrication using plant extracts, (Table 1), 35 g of gold is wet-grinded in 175 g of Cajanus cajan, leaf juice, each day for a period of 15 days after which the metal-cake is dried for 14 days and incinerated. Followed by Ficus racemose, leaf juice (140 g, 12 days), Ricinus communis, leaf juice (105 g, 9 days), Sesbania grandiflora, leaf juice (70 g, 2 days) and finally in 35 g of banana juice for one day. Similarly, for silver ash preparation, 35 g of silver is wet-grinded in 245 g of Solanum nigrum, whole plant juice, each day for a period of 9 days; the cake is dried for 8 days and incinerated. Followed by Datura stramonium, (210 g, 7 days), Ficus racemose (175 g, 5 days), and finally in Chrysanthemum indicum (140 g, 3 days). Incineration is performed after each levigation process using the prescribed amount of heat (Table 1).
The gold ash preparation using mercury was explained in a previous study (8). In brief, purified gold were added to mercury (1:2, ratio), and grinded into amalgam. The amalgam was placed into a clay crucible along with sulphur in a 1:1 ratio and incinerated (~900 °C). The grinding and incineration processes were repeated 42 times. The silver ash preparation method with mercury, used in our previous work (10), is described below. A 4 kg, silver foil, was amalgamated in 1 kg of mercury, and levigated with the Pergularia Daemiar leaf juice, 10 kg. The end product is made into small cakes, dried and incinerated at around 700 °C and the grinding and incineration cycles are repeated two more times. Finally, egg shells 0.5 kg, are added to the incinerated silver and levigated again with the Daemic leaf juice and incinerated repeatedly until a fine powder is obtained.
It is believed that the plants were chosen based on their local and seasonal availability and medicinal properties (Table 1). The antioxidants and the polyols present in the plant extracts act as both reducing and stabilizing agents (62). Most of these plants possess anti-cancerous activity among other notable therapeutic properties (Table 1). It has to be mentioned that the plants used in this fabrication process are today used in the green synthesis of metal nanoparticles (NPs), Table 1. Whether the medicinal properties of the plants are transferred to the final product is controversial because of the high temperatures at which they are incinerated.
The fate of the plant-based components present in the incinerated material is important in order to decide their therapeutic contribution in the final product. The incineration temperature determines the composition of the plant ashes (20). When combusted at temperatures below 450 °C, the main component of the incineration of the plant-based materials will be carbon. As the temperature increases above 450 °C, the carbon becomes volatile and the ash mostly comprises of inorganic carbonates, containing elements such as calcium, magnesium, silicon, sodium, potassium, etc. Above 580 °C, most of the minerals are oxidized, releasing carbon dioxide (63). However, the limited supply of oxygen, might render the carbon compounds incompletely oxidized, to form carbon monoxide. It has to be noted that carbon monoxide is a reducing agent that might have an effect on the oxidation of the metal itself (64). A well finished ash is believed to be devoid of metal remnants or carbonaceous materials (16). Some studies have shown that post-incineration metal ash is devoid of any organic compounds (8,17,21), while others have reported the presence of a carbonaceous material (19,65,66). However, it is suggested that the plants were simply used for their reducing properties and for mechanically easing the grinding process, rather than for their medicinal values.
Further, we think that the inclusion of large amount of plant extract could result in an even distribution of heat during the incineration. In addition, the presence of large amounts of carbon during the repeated incineration processes may have an additional advantage, lowering the vaporization temperature of the metal components. A similar effect of carbon has been found for the gold recovery form sewage sludge. In the absence of carbon, gold is usually released at a temperature around 600 °C and vaporization occurs at 1,000 °C. In the presence of carbon, the gold is released to gas phase at a lower temperature, around 400 °C (67). This temperature is significant, especially when metals such as mercury are present. Given that during the final incineration, the amount of plant components would be much higher when compared with that of the metal itself, the fate of the organic compounds must be probed in those preparations. The combusted plant product contains high amounts of alkaline earth metal oxides that give the trademark whitish color of the ash. The color of pure gold oxide (Au2O3) and silver oxide (Ag2O) is reddish brown and black, respectively (16). The gold and silver ashes (prepared without sulphur) are white powders with a yellow and black tint, respectively.
The metal ashes prepared using mercury are considered superior to the ones that only use plant extracts, probably because the bulk gold or silver will be dispersed into small particles in the mercury matrix, which during incineration results in finer particles (6). The ductility of gold was also observed to be reduced and gold often crystalizes when gold amalgam is subjected to high temperatures (68). The absence of mercury in the ashes were considered as an indication of a high quality incineration (8,20,21) and long incineration times have proved to be beneficial to remove mercury from the ash (8). However, the same cannot be said about all the preparations, a notable amount of mercury is present in many of the metal ashes (17,19,20,24).
Particle size and chemical composition of the meal ashes
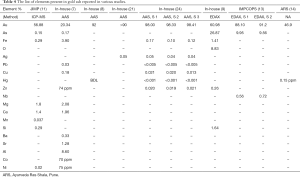
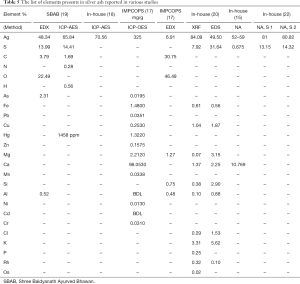
Following the recent development of electron microscopy and X-ray diffraction techniques, the metal ashes were studied for their particle size and chemical composition. Transmission electron microscope (TEM) (8) and scanning electron microscopy (SEM) (11,23) image the morphological appearance of the particles. Dynamic/static light scattering (DLS/SLS), provides the size distribution of the particles. In the case of non-spherical particles such as metal ash, the hydrodynamic radii are analyzed, based on the equivalent sphere (69). Determination of the elemental-chemical composition of the particles was done, using the following methods: Energy Dispersive Spectroscopy (EDS/EDX), X-ray fluorescence (XRF) spectrometry, Inductively coupled plasma mass spectrometry (ICP-MS) and Atomic Absorption Spectroscopy (AAS). The crystalline nature and the crystallite size are determined using X-ray Powder Diffraction (XRD). In the EDX and XRF analysis, the sample is bombarded by electron beam and X-ray, respectively; the X-ray energy emitted is used to identify the elements present. In the ICP-MS, the acid digested metal ashes are plasma ionized, then passed through a quadrupole mass analyser, where they are separated and detected according to their mass-charge ratio (70). In the case of, AAS, the absorption spectrum of the ionized atom is used to detect the elements present in the sample (71). The metal ash is highly heterogeneous in terms of elemental composition and the analysis method plays a huge role in the chemical characterization. Trace elements (concentrations below 0.01 wt%) and the lighter elements (<Na), cannot be detected by EDX. In addition, it has a limited accuracy because the energy peaks overlap among different elements. On the other hand, the irradiation area in XRF is larger than in EDX, and results in better sensitivity when analyzing heterogeneous samples. AAS is reported to quantify elements with better accuracy than the EDX (71), while ICP-MS is more precise than AAS (72). The XRD is used to calculate the crystallite size, and a complimentary method to analyses the chemical composition (13,23). Irrespective of the advantages and limitations presented for the above methods, the studies allowed a closer look at the metal ash and into the fabrication process itself.
In 2007, gold ash was imaged for the first time, by using (TEM) and globular NPs with an average size of 56–57 nm were found (8). In other publications, a mixture of nano and micro size particles was found, Table 2. The NPs were shown to aggregate into particles as large as 5 µm (11). Similar studies showed silver ash to be made up of, nano and micro particles (10,12,15,17-20) (Table 3). The gold content in ash ranged from 50% to 98%, along with varying amounts of other elements (7,8,11,14,15,21,24). The amount of silver also varied between reports 52–81.77% (15,17-19,22). The presence of other elements in the ashes, such as Fe, Zn, Mn, Cu, Ca, etc., was also reported (Tables 4,5). The trace elements are considered a source of the micronutrients required for the body (73).

Full table

Full table
Full table
Full table
The amount silver was found to be reduced with increased number of incineration (22), Table 5. It was reported that after 42 cycles of incineration, the particle size of gold ash was found to be around 56–57 nm (8), nonetheless the particle size in case of lesser or greater number of incineration was not reported. It is known that repeated incinerations of metal particles may result in their aggregation (74). In most methods, the prescribed amount of incineration as shown in the Table 1, is gradually reduced over the consecutive steps. However, there are other methods where the same amount of heat is used throughout the incineration cycles (8). Therefore, the effect of repeated cycles of levigation and incineration is not known. The difference in the size and composition is mostly because of the different fabrication processes (17). In addition, the method used to analyze the metal ash may also have caused the difference in the size and elemental composition (17).
Pre-clinical and clinical analysis of metal ashes
The journey of metal ash inside the body
Unlike the modern nanomedicine, the metal ashes were not designed to target a specific cell type or a receptor. The indications are specified in terms of a particular pathological symptom and, in few cases, a particular organ (40). The pathophysiology belongs to the medieval period, therefore, the drug route, or the pharmacokinetic activity does not coincide with the current understanding. Hence, we can assume that the metal ashes would follow the same fate as any foreign particle that enters the systemic circulation.
In general, before particle enters the systemic circulation, the particle has to pass through the orogastrointestinal tract. The conditions inside the digestive tract are not uniform, the cell type, thickness of the mucus lining, pH, all vary throughout the tract. Immediately after entering the body, the metal oxide NPs are coated by the protein and amino acids (protein corona) in the body fluids. It is reported that the protein corona is formed within 5 min of exposure to the proteins (75). Once consumed, the particle briefly encounters the mucus layer (thickness, 70–100 µm) in the oral cavity and the esophagus and reaches the stomach filled with acidic digestive juices (thickness, 897–1,354 µm) (76). In general, the positively charged, hydrophilic particles and the Zwitterionic particles, have a high chance of penetrating through the mucus layer. On reaching the cell’s surface, in most cases the polarized enterocytes, the particles sterically interact with the cell membrane to gain access into the cell through the transcellular routes. The larger ones, with the particle size >1 µm are taken up by micropinocytosis, ~200 nm by the clathrin- and ~80 nm by caveolin- mediated endocytosis (76). Smaller particle in the size <20 nm may cross the intestinal barrier through the paracellular routes to finally gain access into the systemic circulation. Depending upon, whether the NP is functionalized with specific protein receptors [e.g., Transferrin, g-protein, etc. (77)] to target specific sites, they will circulate all over the body and accumulate in various organs such as liver, spleen, lungs, brain, kidney, testicles, ovaries and skin. The liver is the major clearance routes of the metal oxides >5 nm. They are secreted through bile and are excreted from the body (78). While kidney clears out metal oxides <5 nm through the urine (78), recently it was found that spleen also clears out metal particles from the body.
The analgesic property of gold and silver was for the first time investigated in 1997 using animal models (32-34). Later, numerous studies reported gold ash’s effect on the immune-stimulant activity in brain macrophages (35), as anxiolytic, anti-depressant, anti-cataleptic in behaviorally despaired animals (14,38), free radical scavenging activity in animal models (7), and antioxidant activity in ischemia animal models (36). Similarly, for the silver ash, the sedative-hypnotic activity (26), antibacterial activity (18), anti-inflammatory activity (25), analgesic activity (12), and hypolipidimic activity (37), were reported. The effect of silver ash on brain diseases was also studied (28,30).
Silver ash particles were found to be accumulated in the skin, spleen (malpighian body), liver (histiocytes), kidneys (renal glomerulus), and lymph nodes of the animal models (12). From the above studies, it is clear that the metal ashes were able to cross various barriers and reach the organs. However, the duration of the retention in several organs, the mechanism through which they are excreted from the body is not completely known.
Intercellular fate of the metal ashes
The understanding of how metallic particles behave once they enter the cells is evolving. Most of the particles that enter the cell are processed via. the endosomal-lysosomal system. During the endocytosis process, the particles are encapsulated in a structure called endosome, which gradually transforms into lysosomes. Lysosomes have a highly acidic (pH upto, 4.5) microenvironment that disintegrate the particles and release them into the cell or dispatch them out of the cell via exocytosis (79). Any internalized particle may have the following effect on the cells: they interact with the cell components and induce oxidative stress, disrupt the cytoskeleton, lower the cell proliferation rate, hinder the cell differentiation and on entering the cell nucleus, they may damage the DNA (80). All the above events could eventually lead to the cell death and are exploited for treating diseases like cancer. There are only a few studies exploring the intracellular processing of metal ash particles. Recently, a study on the gold ash cellular uptake confirmed that the particles entered the cells via clathrin-dependent receptor-mediated endocytosis (11). The metal ash particles were accumulated near the peri-nucleus region (10,11). In another study, the uptake and localization of the silver ash particles were found to vary based on the cell types (10). A long term (1 year) retention in vitro study showed that, gold NPs and iron oxide coated gold NPs, were found to disintegrate and recrystallize inside the cells (79,81). The metal ash particles could also follow a similar fate when they are retained in the cells for longer periods.
The metal ashes were considered safe, on the bases that they cannot be metabolized in the body (82), which is not the case. The metal ions are reported to affect various systems- immune (83), nervous (84) and reproductive (85). They release metal ions that generate reactive oxygen species (ROS) in in vivo and in vitro conditions, which plays a key role in the cell survival (86). The ROS cause oxidative stress and affects the cell functions in numerous ways; it interferes with cell-signaling mechanisms and also damages the biomolecules. The DNA damage and inhibition of DNA repair are some of the events that can transform a normal cell into a cancer cell (87). There is a preferential uptake of gold NPs by the cancer cells when compared with normal cells (88). The gold NPs have also shown to cause both necrosis and apoptosis in cancer cells, while only apoptosis in the normal cells. It is not clear if a similar preference is shown by the ash particles and if it is non-toxic for the healthy cells.
Gold ash is reported for their anti-oxidant, free radical scavenging properties (9,36). Another study on gold ash also showed significantly increased superoxide dismutase and catalase activity, two enzymes that reduce free radical concentrations in the body (7). Further, the gold ash was found to be effective in treating various solid malignancies, in particular colon cancer (39). Out of the 39 patients, 17 showed complete (disappearance of all target lesions) or partial response (at least a 30% decrease in the sum of diameters of target lesions), and no significant side effects were observed for the entire duration of 1 years when 50 mg/kg/day was administered. At cell level, gold ash was found to exhibit anti-tumor activity, by disrupting the cytoskeletons of the tumor cells (11).
Toxicity studies on metal ashes
So far, cell-level toxicity studies using MTT assay show that the gold ash is not toxic to both cancer [L929 fibroblast cells (13) and HeLa cells) (11) and non-cancer cells (HFF-1 cells (11)]. Animal study on gold ash have also showed that they do not cause any adverse effect (27). However the gold ash is speculated to have caused adverse effect in a jaundice patient (31). Further, the presence of mercury, arsenic and lead in the gold ashes has also raised concern about their toxic impact in the body (89).
Silver ash was found to lower the lipid levels in the blood, liver and muscles (37), probably due to the impaired lipid metabolism caused by the accumulation of silver ash particles in the liver. In another study, silver ash particles were found aggregated in various organs and a bluish black skin pigmentation was observed in some of the animals (12). These observations highlight the pitfalls in using the silver ashes. Further, the possibility that the gold and silver ash particles could disrupt the tight junctions (13,19) and travel across tight barriers such as BBB is expected to have implication on the brain tissues. It is thought that the adjuvants could prevent the adverse side effects during the administration of metal ashes (90), however there is no study demonstrating the above.
More than one disease can be treated using the same metal by simply varying the adjuvants (91). For example, for shivering, the gold ash is administered along with betel leaf juice, for loss of weight, and discharge of glucose in urine and for rejuvenating effect it is consumed along with honey and butter, respectively (40). There are two hypotheses based on the administration of metal ashes along with adjuvants. The first suggests that, the adjuvants enhance the therapeutic efficiency of the metal ashes and according to the second one; the metal ash increases the therapeutic effect of the phytochemicals present in the adjuvant. A recent study demonstrated that, when gold ash was administered along with pepper (Piper nigrum) and ghee (29), the uptake of the gold ash was enhanced, confirmed by measuring the gold levels in the blood. In order to verify other hypothesis, cell permeability assay on CaCo-2 cell monolayers was performed for the silver ashes. It was found that the silver ash facilitated paracellular transport of AlexaFluor dye molecules, 884.91 g/mol, across the monolayer (19). This study raised the possibility that the metal ashes could have been used to increase the uptake of the phytochemicals present in the plant-based adjuvants. However, in some cases, the metal ashes were consumed simply along warm water and dairy products, which do not have specific pharmacological effect.
Nano-dimension of the metal in the ashes
In the context of the contemporary sciences, the incinerated ashes used in the Indian medicines were repurposed as nanomedicines (6,82,92). Nanomedicines have at least one of their dimensions below 100 nm for ≥50% of the particles (93), or have surface to volume ratios exceeding 60 m2/cm3 or exhibit a physical, chemical and biological effect that attributes to its size, even if it falls outside the nano-range, up to 1,000 nm (94). According to these definitions, the metal ashes are classified as nanomedicine (17). However, not all metal ash particles are <100 nm or <1,000 nm, the percentage of NPs in the metal ash is not uniform (17,95) (Tables 2,3). Gold ash was reported to have only 10% of the particles below 1 µm (24) and for silver ash only <10% of the particles were below 188.7 nm (17). In addition to this, the nano-scale phenomenon are not reported for the metal ashes (2). Probably, due the heterogeneous size distribution or the presence other elements in the metal ashes could mask the nano-size effect. Therefore, it is for reconsideration if the metal ashes can be generalized as nanomedicine.
In a biocompatibility study, unlike metal/metal oxides (75), the gold ash particle did not adsorb proteins, even after 1 h, incubation (13), probably because of the non-uniform shape of the gold ash particles (96). Nonetheless, the therapeutic property of the metal ashes can mainly be attributed to the particle size that favors their cellular uptake (36,97) and their ability to generate ionic species (98). Inspired from Ayurveda, in a recent study, a metal ash-like formulation comprising NPs synthesized through green synthesis and a cocktail of herbal extracts, was reported to be effective on breast cancer patients (89). It is named “nano swarna bashma”, however it is completely different from the gold ash used in Ayurveda, except for the fact that gold is used in both the preparations.
Considering that the major constituents in the metal ashes are the respective metal oxides, the metal ash particles may share similar interactions with the cells and organs as that of the artificially synthesized metal oxide NPs. However, the heterogeneity in size and shape and the presence of an undesirable amount of elements such as mercury, arsenic and lead in the metal ashes may have different therapeutic and toxic implications (99).
Conclusions
In this review, we have summarized the fabrication and therapeutic properties of two important metal-based medicines used in the traditional Indian medicine – gold and silver bhasmas/parpams, also called ashes. The process of fabrication was described, following the ancient texts and methods that are still in use today. However, during the last decades, by using the modern physical methods, the lack of uniformity in terms of composition has been demonstrated for products fabricated by various companies. This is, probably, one of the reasons for the often-contradictory results, regarding the particle-cell interactions. Hence, the scientific validation of these medicines is not conclusive (100); this is a process to be continued and improved once the metal ashes will become more uniform and the analytical methods will progress in terms of accuracy. High quality SEM and TEM measurements of metal-based medicines are required in order to ascertain (or contradict) the assumption, regarding the role of the nano-sized particles in the therapeutic properties of metal-based Ayurvedic medicines. For now, it is difficult to accept the idea that particles incinerated several times at high temperatures would be nano-sized.
There is a tendency among the Indian medicine practitioners to advocate for the safety of these metallic preparations, argue that they are natural products and do not cause any adverse effect (31), or simply because it is an ancient medicine and in practice for many years (101,102). It cannot be denied that the metal ashes were formulated for their safe use and are time tested. The metal ash usage comes with detailed cautionary instructions, about their dosage, adjuvants to be used, anti-dotes in case of adverse effects, dietary advice, etc. (40). However, there is a huge knowledge gap in the understanding their behavior at the cell-level, organ-level and in the entire body. More studies on the sub-cellular behaviors of metal ash particles are required to precisely elucidate their impact at different levels.
Acknowledgments
We thank Dr. M. Krishnaveni, for providing us with clarifications regarding the fabrication of metal ashes.
Funding: The work was support by Horizon Post-doctoral fellowship and NSERC grants of RB and MP.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/lcm-20-34
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/lcm-20-34). MP serves as an unpaid editorial board member of Longhua Chinese Medicine from May 2020 to Apr 2022.The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sravani K, Ketan H, Sanjay S. A Review on Traditional Ayurvedic Preparations Containing Gold. Int J Pharmacogn Phytochem Res 2017;9:801-7.
- Inder D, Kumar P. The Scope of Nano - Silver in Medicine: A Systematic Review. Int J Pharmacogn Chin Med 2018. doi:
10.23880/IPCM-16000134 - Jagtap CY, Patgiri B, Prajapati P, et al. Quality control parameters for Tamra (copper) Bhasma. Anc Sci Life 2012;31:164-70. [Crossref] [PubMed]
- Sujith A, Rajam R, Nechiyil S, et al. Physico-Chemical Characteristics of Munda Loha and Mandoora Bhasmas and Understanding Their Haematinic Effect In Albino Rabbits. Int J Res Ayurveda Pharm 2019;10:112-20. [Crossref]
- Muthukumaran P, Begum VH. Effect of Poorna Chandrodayam Chendooram (PCM-Metallic Drug) on Lipid Profile, Liver Function and Kidney Function Parameters of Rats. Asian J Pharm Anal 2020;10:27-31. [Crossref]
- Kulkarni SS. Bhasma and Nano Medicine. Int Res J Pharm 2016;4:10-6. [Crossref]
- Mitra A, Chakraborty S, Auddy B, et al. Evaluation of chemical constituents and free-radical scavenging activity of Swarnabhasma (gold ash), an ayurvedic drug. J Ethnopharmacol 2002;80:147-53. [Crossref] [PubMed]
- Brown C, Bushell G, Whitehouse MW, et al. Nanogold-pharmaceutics. Gold Bull 2007;40:245-50. [Crossref]
- Sayali C, Girish P. Chemical and Structural Analysis of Ayurvedic Preparation: Swarna Bhasma. Int J Ayu Pharm Chem 2017;6:216-86.
- Parimalam SS, Sohrabi A, Badilescu S, et al. Study of Incinerated Silver Used in Indian Traditional Medicine Systems. Int J Theor Appl Nanotechnol 2020;8:1-7.
- Beaudet D, Badilescu S, Kuruvinashetti K, et al. Comparative study on cellular entry of incinerated ancient gold particles (Swarna Bhasma) and chemically synthesized gold particles. Sci Rep 2017;7:10678. [Crossref] [PubMed]
- Inder D, Rehan HS, Bajaj VK, et al. Analgesic activity and safety of ash of silver used in Indian system of medicine in mice: A reverse pharmacological study. Indian J Pharmacol 2012;44:46-50. [Crossref] [PubMed]
- Paul W, Sharma CP. Blood compatibility studies of Swarna bhasma (gold bhasma), an Ayurvedic drug. Int J Ayurveda Res 2011;2:14-22. [Crossref] [PubMed]
- Bajaj S, Vohora SBB. Anti-cataleptic, anti-anxiety and anti-depressant activity of gold preparations used in Indian systems of medicine. Indian J Pharmacol 2000;32:339-46.
- Galib Barve M. Therapeutic potentials of metals in ancient India: A review through Charaka Samhita. J Ayurveda Integr Med 2011;2:55-63. [Crossref] [PubMed]
- Kapoor RC. Some Observation on The Metal Based Preperations In The Indian System Of Medicine. Indian J Tradit Knowl 2010;9:562-75.
- Sudha A, Murty VS, Chandra TS. Standardization of metal-based herbal medicines. Am J Infect Dis 2009;5:193-9. [Crossref]
- Sharma R, Bhatt A, Thakur M. Physicochemical characterization and antibacterial activity of Rajata Bhasma and silver nanoparticle. Ayu 2016;37:71-5. [Crossref] [PubMed]
- Mukkavalli S, Chalivendra V, Singh BR. Physico-chemical analysis of herbally prepared silver nanoparticles and its potential as a drug bioenhancer. OpenNano 2017;2:19-27. [Crossref]
- Gokarn RA, Kallianpur S, Hebbar K, Madhusudhana K. Characterization of Rajata Bhasma (traditional calcined silver preparation). Int J Green Pharm 2017;11:143-8.
- Ujjal Kumar Sur. Gopa Mandal TG. Physicochemical characterization of Swarna Bhasma: A micro/ nanoparticle used in traditional Indian medicine. Nano Sci Nano Technol 2012;6:104-7.
- Gokarn RA, Patgiri B, Hiremath SG. Pharmaceutical Standardization of Rajatabhasma (Incinerated Silver) by Two Different Methods. Ann Ayurvedic Med 2013;2:7-15.
- Khedekar S. Chemical characterization of incinerated gold (Swarna Bhasma). Adv Appl Sci Res 2015;6:89-95.
- Thakur K, Gudi R, Vahalia M, et al. Preparation and Characterization of Suvarna Bhasma Parada Marit. J Pharmacopuncture 2017;20:36-44. [Crossref] [PubMed]
- Prasad SB. Yashwant, Aeri V. In vitro anti-inflammatory activity of Raupya (Silver) Bhasma. J Chem Pharm Res 2013;5:194-7.
- Inder D, Kumar P. Sedative-hypnotic effect of ash of silver in mice: A reverse pharmacological study. J Tradit Complement Med 2014;4:268-71. [Crossref] [PubMed]
- Jamadagni PS, Jamadagni SB, Singh A, et al. Toxicity study of swarna bhasma, an ayurvedic medicine containing gold, in wistar rats. Toxicol Int 2015;22:11-7. [Crossref]
- Ekka D, Dubey S, Dhruw DS. Effect of Rajat Bhasma with Smritisagar Rasa in Parkinson. J Ayurveda Integr Med Sci 2017;2:146-50.
- Patil-Bhole T, Patil S, Wele AA. Assessment of bioavailability of gold bhasma in human participants - A pilot study. J Ayurveda Integr Med 2018;9:294-7. [Crossref] [PubMed]
- Devi P, Kashyap CP. A clinical evaluation of Rajata Bhasma and Shankhpushpi syrup as medhya. J Ayurvedic Herb Med 2019;5:49-53. [Crossref]
- Paudyal B, Thapa A, Sigdel KR, et al. Adverse events with ayurvedic medicines-possible adulteration and some inherent toxicities. Wellcome Open Res 2019;4:23. [Crossref] [PubMed]
- Sivaraman R, Vohora SB. Research Paper Analgesic Activity of Silver Preparations Used In Indian Systems of Medicine. Drugs 1997;29:393-8.
- Khanna AT, Sivaraman R, Vohora SB. Analgesic activity of silver preparations used in India systems of medicine. Indian J Pharmacol 1997;44:46-50.
- Bajaj S, Vohora SB. Analgesic activity of gold preparations used in Ayurveda and Unani-Tibb. Indian J Med Res 1998;108:104-11. [PubMed]
- Bajaj S, Ahmad I, Raisuddin S, et al. Augmentation of non-specific immunity in mice by gold preparations used in traditional systems of medicine. Indian J Med Res 2001;113:192-6. [PubMed]
- Shah ZA, Vohora SB. Antioxidant/restorative effects of calcined gold preparations used in Indian systems of medicine against global and focal models of ischaemia. Pharmacol Toxicol 2002;90:254-9. [Crossref] [PubMed]
- Sharma DC, Budania R, Shah M, et al. Hypolipidemic activity of silver preparations in chicks, Gallus serregineus. Indian J Exp Biol 2004;42:504-7. [PubMed]
- Shah ZA, Gilani RA, Sharma P, et al. Attenuation of stress-elicited brain catecholamines, serotonin and plasma corticosterone levels by calcined gold preparations used in Indian system of medicine. Basic Clin Pharmacol Toxicol 2005;96:469-74. [Crossref] [PubMed]
- Das S, Das M, Paul R. Swarna Bhasma in cancer: A prospective clinical study. AYU 2012;33:365-7. (An Int Q J Res Ayurveda). [Crossref] [PubMed]
- Thiagarajan R, Gunapadam-Thathu Jeeva vaguppu. 4th edition. Directorate of Indian. 2004.
- Ashokkumar T, Prabhu D, Geetha R, et al. Apoptosis in liver cancer (HepG2) cells induced by functionalized gold nanoparticles. Colloids Surfaces B Biointerfaces 2014;123:549-56. [Crossref] [PubMed]
- Pal D, Mishra P, Sachan N, et al. Biological activities and medicinal properties of Cajanus cajan (L) Millsp. J Adv Pharm Technol Res 2011;2:207-14. [Crossref] [PubMed]
- Tetgure SR, Borse AU, Sankapal BR, et al. Green biochemistry approach for synthesis of silver and gold nanoparticles using Ficus racemosa latex and their pH-dependent binding study with different amino acids using UV/Vis absorption spectroscopy. Amino Acids 2015;47:757-65. [Crossref] [PubMed]
- Yadav RK, Nandy BC, Maity S, et al. Phytochemistry, pharmacology, toxicology, and clinical trial of Ficus racemosa. Pharmacogn Rev 2015;9:73-80. [Crossref] [PubMed]
- Ghramh HA, Khan KA, Ibrahim EH, et al. Synthesis of gold nanoparticles (AuNPs) using ricinus communis leaf ethanol extract, their characterization, and biological applications. Nanomaterials 2019;9:765. [Crossref] [PubMed]
- Abdul W, Hajrah N, Sabir J, et al. Therapeutic role of Ricinus communis L. and its bioactive compounds in disease prevention and treatment. Asian Pac J Trop Med 2018;11:177-85. [Crossref]
- Rajendran SP, Sengodan K. Synthesis and Characterization of Zinc Oxide and Iron Oxide Nanoparticles Using Sesbania grandiflora Leaf Extract as Reducing Agent. J Nanosci 2017;17:1-7.
- Pajaniradje S, Mohankumar K, Pamidimukkala R, et al. Antiproliferative and apoptotic effects of sesbania grandiflora leaves in human cancer cells. Biomed Res Int 2014;2014:474953 [Crossref] [PubMed]
- Ibrahim HMM. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci 2015;8:265-75. [Crossref]
- Ado Ahmad B, Abdullahi Zakariyya U, Abubakar M, et al. Pharmacological Activities of Banana. In: Banana Nutrition - Function and Processing Kinetics. 2020;1-20.
- Muthuvel A, Adavallan K, Balamurugan K, et al. Biosynthesis of gold nanoparticles using Solanum nigrum leaf extract and screening their free radical scavenging and antibacterial properties. Biomed Prev Nutr 2014;4:325-32. [Crossref]
- Lai YJ, Tai CJ, Wang CW, et al. Anti-cancer activity of Solanum nigrum (AESN) through suppression of mitochondrial function and epithelial-mesenchymal transition (EMT) in breast cancer cells. Molecules 2016;21:553. [Crossref] [PubMed]
- Oladipo IC, Lateef A, Azeez MA, et al. Characterization and biomedical application of phytosynthesized gold nanoparticles from Datura stramonium seed extract. IOP Conference Series: Materials Science and Engineering 2020;805:012021 [Crossref]
- Soni P, Siddiqui AA, Dwivedi J, et al. Pharmacological properties of Datura stramonium L. as a potential medicinal tree: An overview. Asian Pac J Trop Biomed 2012;2:1002-8. [Crossref] [PubMed]
- Arokiyaraj S, Arasu MV, Vincent S, et al. Rapid green synthesis of silver nanoparticles from chrysanthemum indicum land its antibacterial and cytotoxic effects: An in vitro study. Int J Nanomedicine 2014;9:379-88. [Crossref] [PubMed]
- Kim C, Kim MC, Kim SM, et al. Chrysanthemum indicum L. extract induces apoptosis through suppression of constitutive STAT3 activation in human prostate cancer DU145 cells. Phytother Res 2013;27:30-8. [Crossref] [PubMed]
- Anjali S, Rajam R, Nechiyil S, et al. Physico-Chemical Characteristics of Munda Loha and Mandoora Bhasma and Understanding their Haematinic Effect in Albino Rabbits. Int J Res Ayurveda Pharm 2019;10:112-20. [Crossref]
- Chaturvedi R, Jha C. Standard manufacturing procedure of Rajata Bhasma. Ayu 2011;32:566-71. [Crossref] [PubMed]
- Randhawa GK, Sharma R. Chemotherapeutic potential of cow urine: A review. J Intercult Ethnopharmacol 2015;4:180-6. [Crossref] [PubMed]
- Chelgani SC, Parian M, Parapari PS, et al. A comparative study on the effects of dry and wetgrinding on mineral flotation separation-a review. J Mater Res Technol 2019;8:5004-11. [Crossref]
- Braadbaart F, Poole I, Huisman HDJ, et al. Fuel, Fire and Heat: An experimental approach to highlight the potential of studying ash and char remains from archaeological contexts. J Archaeol Sci 2012;39:836-47. [Crossref]
- Mohamad NAN, Arham NA, Jai J, et al. Plant extract as reducing agent in synthesis of metallic nanoparticles: A review. Advanced Materials Research 2014;832:350-5. [Crossref]
- Justice A, Roggen M. What Is the Science Behind White Ash and Black Ash? [Internet] 2019 [cited 2020 Aug 13]. Available online: https://www.cannabisbusinesstimes.com/article/white-ash-vs-black-ash/
- Tsygankov AA, Makarova M, Chusov D. Carbon monoxide as a selective reducing agent in organic chemistry. Mendeleev Commun 2018;28:113-22. [Crossref]
- Singh SK, Rai SB. Detection of carbonaceous material in Naga Bhasma. Indian J Pharm Sci 2012;74:178-83. [Crossref] [PubMed]
- Thornton MA, Thomas TH, Peters NCB. The promotive effect of combustion products from plant vegetation on the release of seeds from dormancy. Plant Growth Regul 1999;28:129-32. [Crossref]
- Sugawara K, Sakusabe K, Kato T, et al. Selective Recovery of Gold from Incinerated Sewage Sludge Ash. J Soc Powder Technol Japan 2016;2:2297-300.
- Michaud LD. Solubility of Gold [Internet] 2016 [cited 2020 Aug 13]. Available online: https://www.911metallurgist.com/blog/solubility-of-gold
- Bootz A, Vogel V, Schubert D, et al. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur J Pharm Biopharm 2004;57:369-75. [Crossref] [PubMed]
- Wilschefski SC, Baxter MR. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin Biochem Rev 2019;40:115-33. [Crossref] [PubMed]
- Modi AT, Kjonstad TA, White BJ. Comparison of EDX and AA to determine distribution of Ca2+ in Phaseolus vulgaris (L.) seed parts following osmopriming. South African J Bot 2004;70:298-302. [Crossref]
- Akinyele IO, Shokunbi OS. Comparative analysis of dry ashing and wet digestion methods for the determination of trace and heavy metals in food samples. Food Chem 2015;173:682-4. [Crossref] [PubMed]
- Dongre D. Sushma KDKD. Metal Bhasmas: A Possible Sourceof Trace Elements. Int J Ayurveda Pharma Res 2016;4:72-4.
- Catauro M, Tranquillo E, Dal Poggetto G, et al. Influence of the heat treatment on the particles size and on the crystalline phase of TiO2 synthesized by the sol-gel method. Materials (Basel) 2018;11:2364. [Crossref]
- Bergin IL, Witzmann FA. Nanoparticle toxicity by the gastrointestinal route: evidence and knowledge gaps. Int J Biomed Nanosci Nanotechnol 2013;3:054515 [Crossref] [PubMed]
- Fröhlich E, Roblegg E. Models for oral uptake of nanoparticles in consumer products. Toxicology 2012;291:10-7. [Crossref] [PubMed]
- Yoo J, Park C, Yi G, et al. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers (Basel) 2019;11:640-53. [Crossref] [PubMed]
- Yao Y, Zang Y, Qu J, et al. The toxicity of metallic nanoparticles on liver: The subcellular damages, mechanisms, and outcomes. Int J Nanomedicine 2019;14:8787-804. [Crossref] [PubMed]
- Balfourier A, Luciani N, Wang G, et al. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc Natl Acad Sci U S A 2020;117:103-13. [Crossref] [PubMed]
- Kaur P, Aliru ML, Chadha AS, et al. Hyperthermia using nanoparticles - Promises and pitfalls. Int J Hyperthermia 2016;32:76-88. [Crossref] [PubMed]
- Kolosnjaj-Tabi J, Javed Y, et al. The One Year Fate of Iron Oxide Coated Gold Nanoparticles in Mice. ACS Nano 2015;9:7925-39. [Crossref] [PubMed]
- Pal D, Sahu CK, Haldar A. Bhasma: The ancient Indian nanomedicine. J Adv Pharm Technol Res 2014;5:4-12. [Crossref] [PubMed]
- Lehmann I, Sack U, Lehmann J. Metal ions affecting the immune system. Met Ions Life Sci 2011;8:157-85. [PubMed]
- Pohl HR, Roney N, Abadin HG. Metal ions affecting the neurological system. Met Ions Life Sci 2011;8:247-62. [PubMed]
- Apostoli P, Catalani S. Metal ions affecting reproduction and development. Met Ions Life Sci 2011;8:263-303. [PubMed]
- Shrivastava R, Kushwaha P, Bhutia YC, et al. Oxidative stress following exposure to silver and gold nanoparticles in mice. Toxicol Ind Health 2016;32:1391-404. [Crossref] [PubMed]
- Xu J, Wise JTF, Wang L, et al. Dual roles of oxidative stress in metal carcinogenesis. J Environ Pathol Toxicol Oncol 2017;36:345-76. [Crossref] [PubMed]
- Xia Q, Huang J, Feng Q, et al. Size- and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. Int J Nanomedicine 2019;14:6957-70. [Crossref] [PubMed]
- Khoobchandani M, Katti KK, Karikachery AR, et al. New approaches in breast cancer therapy through green nanotechnology and nano-ayurvedic medicine - pre-clinical and pilot human clinical investigations. Int J Nanomedicine 2020;15:181-97. [Crossref] [PubMed]
- Kapoor RC. Some observations on the metal-based preparations in the Indian Systems of Medicine. Indian J Tradit Knowl 2010;9:562-75.
- Chaturvedi R, Bhargava SC, Sonkar N, et al. Rajata in Ayurvedic therapeutics. Biomed Pharmacol J 2009;2:407-16.
- Sharma R, Prajapati P. Nanotechnology in medicine: Leads from Ayurveda. J Pharm Bioallied Sci 2016;8:80-1. [Crossref] [PubMed]
- Soares S, Sousa J, Pais A, Vitorino C. Nanomedicine: Principles, properties, and regulatory issues. Front Chem 2018;6:360. [Crossref] [PubMed]
- Satalkar P, Elger BS, Shaw DM. Defining Nano, Nanotechnology and Nanomedicine: Why Should It Matter? Sci Eng Ethics 2016;22:1255-76. [Crossref] [PubMed]
- Rathore M, Joshi DS, Kadam SN, et al. Swarna Bhasmas do contain nanoparticles? Int J Pharma Bio Sci 2013;4:243-9.
- Wang G, Wang W, Shangguan E, et al. Effects of gold nanoparticle morphologies on interactions with proteins. Mater Sci Eng C Mater Biol Appl 2020;111:110830 [Crossref] [PubMed]
- Rai AP, Tripathi S, Tiwari OP. Assessment of hepatoprotective activity of Rajata bhasma in CCl4 induced hepatotoxicity rats. Biomed Pharmacol J 2019;12:1731-5. [Crossref]
- Chaudhary RG, Bhusari GS, Tiple AD, et al. Metal/Metal Oxide Nanoparticles: Toxicity, Applications, and Future Prospects. Curr Pharm Des 2019;25:4013-29. [Crossref] [PubMed]
- Mikulski MA, Wichman MD, Simmons DL, et al. Toxic metals in ayurvedic preparations from a public health lead poisoning cluster investigation. Int J Occup Environ Health 2017;23:187-92. [Crossref] [PubMed]
- Chandramouli R, Thirunarayanan T, Mukeshbabu K, et al. Designing toxicological evaluation of ayurveda and siddha products to cater to global compliance - current practical and regulatory perspectives. J Pharm Sci Res 2010;2:867-77.
- Liu J, Zhang F, Ravikanth V, et al. Chemical Compositions of Metals in Bhasmas and Tibetan Zuotai Are a Major Determinant of Their Therapeutic Effects and Toxicity. Evid Based Complement Alternat Med 2019;2019:1697804 [PubMed]
- Kessler C, Wischnewsky M, Michalsen A, et al. Ayurveda: Between religion, spirituality, and medicine. Evid Based Complement Alternat Med 2013;2013:952432 [Crossref] [PubMed]
Cite this article as: Subramaniyan Parimalam S, Badilescu S, Bhat R, Packirisamy M. A Narrative review of scientific validation of gold- and silver-based Indian medicines and their future scope. Longhua Chin Med 2020;3:10.

